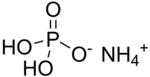
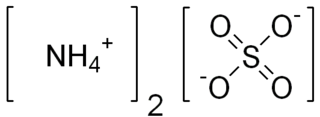In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid, a.k.a. phosphoric acid H3PO4.
Urea, also called carbamide, is an organic compound with chemical formula CO(NH2)2. This amide has two amino groups joined by a carbonyl functional group. It is thus the simplest amide of carbamic acid.

A fertilizer or fertiliser is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from liming materials or other non-nutrient soil amendments. Many sources of fertilizer exist, both natural and industrially produced. For most modern agricultural practices, fertilization focuses on three main macro nutrients: nitrogen (N), phosphorus (P), and potassium (K) with occasional addition of supplements like rock flour for micronutrients. Farmers apply these fertilizers in a variety of ways: through dry or pelletized or liquid application processes, using large agricultural equipment or hand-tool methods.

Phosphoric acid is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula H3PO4. It is commonly encountered as an 85% aqueous solution, which is a colourless, odourless, and non-volatile syrupy liquid. It is a major industrial chemical, being a component of many fertilizers.
The nitrophosphate process is a method for the industrial production of nitrogen fertilizers invented by Erling Johnson in the municipality of Odda, Norway around 1927.
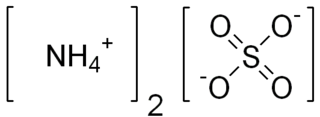
Ammonium sulfate (American English and international scientific usage; ammonium sulphate in British English); (NH4)2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen and 24% sulfur.

Monopotassium phosphate (MKP) (also, potassium dihydrogen phosphate, KDP, or monobasic potassium phosphate) is the inorganic compound with the formula KH2PO4. Together with dipotassium phosphate (K2HPO4.(H2O)x) it is often used as a fertilizer, food additive, and buffering agent. The salt often cocrystallizes with the dipotassium salt as well as with phosphoric acid.

Diammonium phosphate (DAP; IUPAC name diammonium hydrogen phosphate; chemical formula (NH4)2(HPO4) is one of a series of water-soluble ammonium phosphate salts that can be produced when ammonia reacts with phosphoric acid.

In chemistry, a phosphoric acid, in the general sense, is a phosphorus oxoacid in which each phosphorus (P) atom is in the oxidation state +5, and is bonded to four oxygen (O) atoms, one of them through a double bond, arranged as the corners of a tetrahedron. Two or more of these PO4 tetrahedra may be connected by shared single-bonded oxygens, forming linear or branched chains, cycles, or more complex structures. The single-bonded oxygen atoms that are not shared are completed with acidic hydrogen atoms. The general formula of a phosphoric acid is Hn+2−2xPnO3n+1−x, where n is the number of phosphorus atoms and x is the number of fundamental cycles in the molecule's structure, between 0 and n + 2/2.

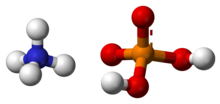
Ammonium phosphate is the inorganic compound with the formula (NH4)3PO4. It is the ammonium salt of orthophosphoric acid. A related "double salt", (NH4)3PO4.(NH4)2HPO4 is also recognized but is impractical to use. Both triammonium salts evolve ammonia. In contrast to the unstable nature of the triammonium salts, the diammonium phosphate (NH4)2HPO4 and monoammonium salt (NH4)H2PO4 are stable materials that are commonly used as fertilizers to provide plants with fixed nitrogen and phosphorus.
In chemistry, phosphorus oxoacid is a generic name for any acid whose molecule consists of atoms of phosphorus, oxygen, and hydrogen. There is a potentially infinite number of such compounds. Some of them are unstable and have not been isolated, but the derived anions and organic groups are present in stable salts and esters. The most important ones—in biology, geology, industry, and chemical research—are the phosphoric acids, whose esters and salts are the phosphates.

Struvite (magnesium ammonium phosphate) is a phosphate mineral with formula: NH4MgPO4·6H2O. Struvite crystallizes in the orthorhombic system as white to yellowish or brownish-white pyramidal crystals or in platy mica-like forms. It is a soft mineral with Mohs hardness of 1.5 to 2 and has a low specific gravity of 1.7. It is sparingly soluble in neutral and alkaline conditions, but readily soluble in acid.

Monocalcium phosphate is an inorganic compound with the chemical formula Ca(H2PO4)2 ("AMCP" or "CMP-A" for anhydrous monocalcium phosphate). It is commonly found as the monohydrate ("MCP" or "MCP-M"), Ca(H2PO4)2·H2O. Both salts are colourless solids. They are used mainly as superphosphate fertilizers and are also popular leavening agents.

Dihydrogen phosphate is an inorganic ion with the formula [H2PO4]−. Phosphates occur widely in natural systems.

Monosodium phosphate (MSP), also known as monobasic sodium phosphate and sodium dihydrogen phosphate, is an inorganic compound with the chemical formula NaH2PO4. It is a sodium salt of phosphoric acid. It consists of sodium cations (Na+) and dihydrogen phosphate anions (H2PO−4). One of many sodium phosphates, it is a common industrial chemical. The salt exists in an anhydrous form, as well as monohydrate and dihydrate (NaH2PO4·H2O and NaH2PO4·2H2O respectively).

Mangalore Chemicals & Fertilizers Limited is the largest manufacturer of chemical fertilizers in the state of Karnataka, India. The company is part of the Adventz Group. The company's corporate and registered office is at UB City, Bangalore and its factory unit is in Panambur, north of Mangalore.

Ammonium polyphosphate is an inorganic salt of polyphosphoric acid and ammonia containing both chains and possibly branching. Its chemical formula is H(NH4PO3)nOH showing that each monomer consists of an orthophosphate radical of a phosphorus atom with three oxygens and one negative charge neutralized by an ammonium cation leaving two bonds free to polymerize. In the branched cases some monomers are missing the ammonium anion and instead link to three other monomers.
Commodity chemicals are a group of chemicals that are made on a very large scale to satisfy global markets. The average prices of commodity chemicals are regularly published in the chemical trade magazines and web sites such as Chemical Week and ICIS. There have been several studies of the scale and complexity of this market for example in the USA.
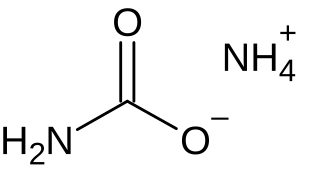
Ammonium carbamate is a chemical compound with the formula [NH4][H2NCO2] consisting of ammonium cation NH+4 and carbamate anion NH2COO−. It is a white solid that is extremely soluble in water, less so in alcohol. Ammonium carbamate can be formed by the reaction of ammonia NH3 with carbon dioxide CO2, and will slowly decompose to those gases at ordinary temperatures and pressures. It is an intermediate in the industrial synthesis of urea (NH2)2CO, an important fertilizer.