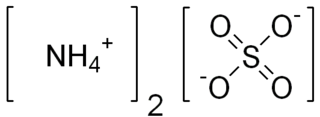Bromomethane, commonly known as methyl bromide, is an organobromine compound with formula CH3Br. This colorless, odorless, nonflammable gas is produced both industrially and biologically. It has a tetrahedral shape and it is a recognized ozone-depleting chemical. It was used extensively as a pesticide until being phased out by most countries in the early 2000s.

1,2-Dibromoethane, also known as ethylene dibromide (EDB), is an organobromine compound with the chemical formula C
2H
4Br
2. Although trace amounts occur naturally in the ocean, where it is formed probably by algae and kelp, it is mainly synthetic. It is a dense colorless liquid with a faint sweet odor, detectable at 10 ppm, is a widely used and sometimes-controversial fumigant. The combustion of 1,2-dibromoethane produces hydrogen bromide gas that is significantly corrosive.

Barium nitrate is the inorganic compound with the chemical formula Ba(NO3)2. It, like most barium salts, is colorless, toxic, and water-soluble. It burns with a green flame and is an oxidizer; the compound is commonly used in pyrotechnics.

The term immediately dangerous to life or health (IDLH) is defined by the US National Institute for Occupational Safety and Health (NIOSH) as exposure to airborne contaminants that is "likely to cause death or immediate or delayed permanent adverse health effects or prevent escape from such an environment." Examples include smoke or other poisonous gases at sufficiently high concentrations. It is calculated using the LD50 or LC50. The Occupational Safety and Health Administration (OSHA) regulation defines the term as "an atmosphere that poses an immediate threat to life, would cause irreversible adverse health effects, or would impair an individual's ability to escape from a dangerous atmosphere."
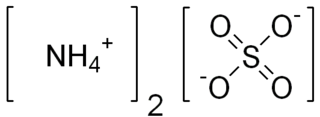
Ammonium sulfate (American English and international scientific usage; ammonium sulphate in British English); (NH4)2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen and 24% sulfur.

Toluene diisocyanate (TDI) is an organic compound with the formula CH3C6H3(NCO)2. Two of the six possible isomers are commercially important: 2,4-TDI (CAS: 584-84-9) and 2,6-TDI (CAS: 91-08-7). 2,4-TDI is produced in the pure state, but TDI is often marketed as 80/20 and 65/35 mixtures of the 2,4 and 2,6 isomers respectively. It is produced on a large scale, accounting for 34.1% of the global isocyanate market in 2000, second only to MDI. Approximately 1.4 billion kilograms were produced in 2000. All isomers of TDI are colorless, although commercial samples can appear yellow.

Phosphorus trichloride is a inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic and reacts readily with water to release hydrogen chloride.
Chloropicrin, also known as PS and nitrochloroform, is a chemical compound currently used as a broad-spectrum antimicrobial, fungicide, herbicide, insecticide, and nematicide. Its chemical structural formula is Cl3CNO2.

Chlorobenzene is an aromatic organic compound with the chemical formula C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals.
Hydrogen selenide is an inorganic compound with the formula H2Se. This hydrogen chalcogenide is the simplest and most commonly encountered hydride of selenium. H2Se is a colorless, flammable gas under standard conditions. It is the most toxic selenium compound with an exposure limit of 0.05 ppm over an 8-hour period. Even at extremely low concentrations, this compound has a very irritating smell resembling that of decayed horseradish or 'leaking gas', but smells of rotten eggs at higher concentrations.

Aldrin is an organochlorine insecticide that was widely used until the 1990s, when it was banned in most countries. Aldrin is a member of the so-called "classic organochlorines" (COC) group of pesticides. COCs enjoyed a very sharp rise in popularity during and after The Second World War. Other noteworthy examples of COCs include DDT. After research showed that organochlorines can be highly toxic to the ecosystem through bioaccumulation, most were banned from use. It is a colourless solid. Before the ban, it was heavily used as a pesticide to treat seed and soil. Aldrin and related "cyclodiene" pesticides became notorious as persistent organic pollutants.

Thallium(I) sulfate (Tl2SO4) or thallous sulfate is the sulfate salt of thallium in the common +1 oxidation state, as indicated by the Roman numeral I. It is often referred to as simply thallium sulfate.

Picloram is a systemic herbicide used for general woody plant control. It also controls a wide range of broad-leaved weeds, but most grasses are resistant. A chlorinated derivative of picolinic acid, picloram is in the pyridine family of herbicides.

Methoxychlor is a synthetic organochloride insecticide, now obsolete.

Calcium arsenate is the inorganic compound with the formula Ca3(AsO4)2. A colourless salt, it was originally used as a pesticide and as a germicide. It is highly soluble in water, as compared with lead arsenate, which makes it more toxic. The minerals rauenthalite Ca3(AsO4)2·10H2O and phaunouxite Ca3(AsO4)2·11H2O are hydrates of calcium arsenate.
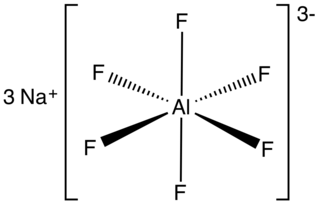
Sodium aluminium hexafluoride is an inorganic compound with formula Na3AlF6. This white solid, discovered in 1799 by Peder Christian Abildgaard (1740–1801), occurs naturally as the mineral cryolite and is used extensively in the industrial production of aluminium metal. The compound is the sodium (Na+) salt of the hexafluoroaluminate (AlF63−) ion.

Dichlorvos is an organophosphate widely used as an insecticide to control household pests, in public health, and protecting stored products from insects. The compound has been commercially available since 1961 and has become controversial because of its prevalence in urban waterways and the fact that its toxicity extends well beyond insects. The insecticide has been banned in EU since 1998.

n-Butylamine is an organic compound (specifically, an amine) with the formula CH3(CH2)3NH2. This colourless liquid is one of the four isomeric amines of butane, the others being sec-butylamine, tert-butylamine, and isobutylamine. It is a liquid having the fishy, ammonia-like odor common to amines. The liquid acquires a yellow color upon storage in air. It is soluble in all organic solvents. Vapors heavier than air and it produces toxic oxides of nitrogen during combustion.

Thiram is the simplest thiuram disulfide and the oxidized dimer of dimethyldithiocarbamate. It is used as a fungicide, ectoparasiticide to prevent fungal diseases in seed and crops and similarly as an animal repellent to protect fruit trees and ornamentals from damage by rabbits, rodents and deer. It is effective against Stem gall of coriander, damping off, smut of millet, neck rot of onion, etc. Thiram has been used in the treatment of human scabies, as a sun screen and as a bactericide applied directly to the skin or incorporated into soap.
ortho-Cresol, also called 2-methylphenol, or 2-hydroxytoluene, is an organic compound with the formula CH3C6H4(OH). It is a colourless solid that is widely used intermediate in the production of other chemicals. It is a derivative of phenol and is an isomer of p-cresol and m-cresol.