
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula NH3. A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and diammonium phosphate. Ammonia in pure form is also applied directly into the soil.

Sodium fluoride (NaF) is an inorganic compound with the formula NaF. It is a colorless or white solid that is readily soluble in water. It is used in trace amounts in the fluoridation of drinking water to prevent tooth decay, and in toothpastes and topical pharmaceuticals for the same purpose. In 2020, it was the 265th most commonly prescribed medication in the United States, with more than 1 million prescriptions. It is also used in metallurgy and in medical imaging.
Boron trifluoride is the inorganic compound with the formula BF3. This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.

Cadmium chloride is a white crystalline compound of cadmium and chloride, with the formula CdCl2. This salt is a hygroscopic solid that is highly soluble in water and slightly soluble in alcohol. The crystal structure of cadmium chloride (described below), is a reference for describing other crystal structures. Also known are CdCl2•H2O and the hemipenahydrate CdCl2•2.5H2O.

Sodium peroxide is an inorganic compound with the formula Na2O2. This yellowish solid is the product of sodium ignited in excess oxygen. It is a strong base. This metal peroxide exists in several hydrates and peroxyhydrates including Na2O2·2H2O2·4H2O, Na2O2·2H2O, Na2O2·2H2O2, and Na2O2·8H2O. The octahydrate, which is simple to prepare, is white, in contrast to the anhydrous material.

Tiabendazole, also known as thiabendazole or TBZ and the trade names Mintezol, Tresaderm, and Arbotect, is a preservative, an antifungal agent, and an antiparasitic agent.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide.

Iron(III) fluoride, also known as ferric fluoride, are inorganic compounds with the formula FeF3(H2O)x where x = 0 or 3. They are mainly of interest by researchers, unlike the related iron(III) chloride. Anhydrous iron(III) fluoride is white, whereas the hydrated forms are light pink.
Antimony pentafluoride is the inorganic compound with the formula SbF5. This colourless, viscous liquid is a strong Lewis acid and a component of the superacid fluoroantimonic acid, formed upon mixing liquid HF with liquid SbF5 in 1:1 ratio. It is notable for its strong Lewis acidity and the ability to react with almost all known compounds.
Tellurium hexafluoride is the inorganic compound of tellurium and fluorine with the chemical formula TeF6. It is a colorless, highly toxic gas with an unpleasant odor.
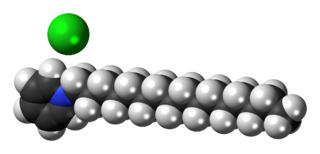
Cetylpyridinium chloride (CPC) is a cationic quaternary ammonium compound used in some types of mouthwashes, toothpastes, lozenges, throat sprays, breath sprays, and nasal sprays. It is an antiseptic that kills bacteria and other microorganisms. It has been shown to be effective in preventing dental plaque and reducing gingivitis. It has also been used as an ingredient in certain pesticides. Though one study seems to indicate cetylpyridinium chloride does not cause brown tooth stains, at least one mouthwash containing CPC as an active ingredient bears the warning label "In some cases, antimicrobial rinses may cause surface staining to teeth," following a failed class-action lawsuit brought by customers whose teeth were stained.

Silver sulfate is the inorganic compound with the formula Ag2SO4. It is a white solid with low solubility in water.
The Balz–Schiemann reaction is a chemical reaction in which a primary aromatic amine is transformed to an aryl fluoride via a diazonium tetrafluoroborate intermediate. This reaction is a traditional route to fluorobenzene and some related derivatives, including 4-fluorobenzoic acid.

Hexafluorophosphate is an anion with chemical formula of [PF6]−. It is an octahedral species that imparts no color to its salts. [PF6]− is isoelectronic with sulfur hexafluoride, SF6, and the hexafluorosilicate dianion, [SiF6]2−, and hexafluoroantimonate [SbF6]−. In this anion, phosphorus has a valence of 5. Being poorly nucleophilic, hexafluorophosphate is classified as a non-coordinating anion.
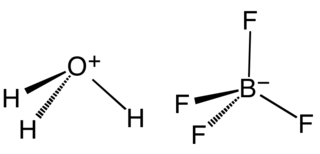
Fluoroboric acid or tetrafluoroboric acid is an inorganic compound with the simplified chemical formula H+[BF4]−. Unlike other strong acids like H2SO4 or HClO4, the pure tetrafluoroboric acid does not exist. The term "fluoroboric acid" refers to a range of chemical compounds, depending on the solvent. The H+ in the simplified formula of fluoroboric acid represents the solvated proton. The solvent can be any suitable Lewis base. For instance, if the solvent is water, fluoroboric acid can be represented by the formula [H3O]+[BF4]−, although more realistically, several water molecules solvate the proton: [H(H2O)n]+[BF4]−. The ethyl ether solvate is also commercially available, where the fluoroboric acid can be represented by the formula [H( 2O)n]+[BF4]−, where n is most likely 2.
Sodium tetrafluoroborate is an inorganic compound with formula NaBF4. It is a salt that forms colorless or white water-soluble rhombic crystals and is soluble in water (108 g/100 mL) but less soluble in organic solvents.

α-Naphthylthiourea (ANTU) is an organosulfur compound with the formula C10H7NHC(S)NH2. This a white, crystalline powder although commercial samples may be off-white. It is used as a rodenticide and as such is fairly toxic. Naphthylthiourea is available as 10% active baits in suitable protein- or carbohydrate-rich materials and as a 20% tracking powder.

Diacetyl peroxide is the organic peroxide with the formula (CH3CO2)2. It is a white solid or oily liquid with a sharp odor. As with a number of organic peroxides, it is explosive. It is often used as a solution, e.g., in dimethyl phthalate.

Cadmium tetrafluoroborate is an ionic, chemical compound with the formula Cd(BF4)2. It is a crystalline solid, which is colorless and odorless. Cadmium tetrafluoroborate is most frequently used in the industrial production of high-strength steels, its purpose being to prevent hydrogen absorption, a source of post-production cracking of the metal, in the treated steels. Another application of the chemistry of cadmium tetrafluoroborate is fine tuning of the size of cadmium telluride nanomaterials.
Europium(III) fluoride is an inorganic compound with a chemical formula EuF3.


















