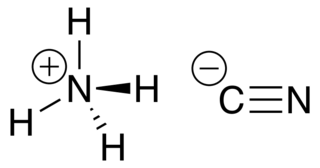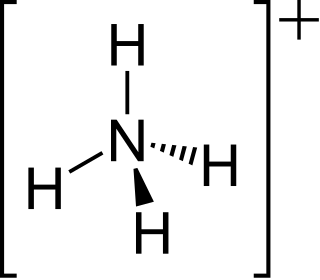
The ammonium cation is a positively charged polyatomic ion with the chemical formula NH+4 or [NH4]+. It is formed by the protonation of ammonia. Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations, where one or more hydrogen atoms are replaced by organic or other groups.

Hydroxylamine is an inorganic compound with the chemical formula NH2OH. The compound is in a form of a white hygroscopic crystals. Hydroxylamine is almost always provided and used as an aqueous solution. It is consumed almost exclusively to produce Nylon-6. The oxidation of NH3 to hydroxylamine is a step in biological nitrification.

Zinc chloride is the name of inorganic chemical compounds with the formula ZnCl2·nH2O, with x ranging from 0 to 4.5, forming hydrates. Zinc chloride, anhydrous and its hydrates are colorless or white crystalline solids, and are highly soluble in water. Five hydrates of zinc chloride are known, as well as four forms of anhydrous zinc chloride. This salt is hygroscopic and even deliquescent. Zinc chloride finds wide application in textile processing, metallurgical fluxes, and chemical synthesis. No mineral with this chemical composition is known aside from the very rare mineral simonkolleite, Zn5(OH)8Cl2·H2O.

Ceric ammonium nitrate (CAN) is the inorganic compound with the formula (NH4)2[Ce(NO3)6]. This orange-red, water-soluble cerium salt is a specialised oxidizing agent in organic synthesis and a standard oxidant in quantitative analysis.

A permanganate is a chemical compound with the manganate(VII) ion, MnO−
4, the conjugate base of permanganic acid. Because the manganese atom has a +7 oxidation state, the permanganate(VII) ion is a strong oxidising agent. The ion is a transition metal ion with a tetrahedral structure. Permanganate solutions are purple in colour and are stable in neutral or slightly alkaline media. The exact chemical reaction depends on the carbon-containing reactants present and the oxidant used. For example, trichloroethane (C2H3Cl3) is oxidised by permanganate ions to form carbon dioxide (CO2), manganese dioxide (MnO2), hydrogen ions (H+), and chloride ions (Cl−).

Peroxymonosulfuric acid, H
2SO
5, is also known as persulfuric acid, peroxysulfuric acid, or Caro's acid. In this acid, the S(VI) center adopts its characteristic tetrahedral geometry; the connectivity is indicated by the formula HO–O–S(O)2–OH. It is one of the strongest oxidants known (E0 = +2.51 V) and is highly explosive.

Ammonium nitrite, [NH4]NO2, is the ammonium salt of nitrous acid. It is not used in pure isolated form since it is highly unstable and decomposes into water and nitrogen, even at room temperature.
A persulfate is a compound containing the anions SO2−
5 or S
2O2−
8. The anion SO2−
5 contains one peroxide group per sulfur center, whereas in S
2O2−
8, the peroxide group bridges the sulfur atoms. In both cases, sulfur adopts the normal tetrahedral geometry typical for the S(VI) oxidation state. These salts are strong oxidizers.

Terbium(III,IV) oxide, occasionally called tetraterbium heptaoxide, has the formula Tb4O7, though some texts refer to it as TbO1.75. There is some debate as to whether it is a discrete compound, or simply one phase in an interstitial oxide system. Tb4O7 is one of the main commercial terbium compounds, and the only such product containing at least some Tb(IV) (terbium in the +4 oxidation state), along with the more stable Tb(III). It is produced by heating the metal oxalate, and it is used in the preparation of other terbium compounds. Terbium forms three other major oxides: Tb2O3, TbO2, and Tb6O11.
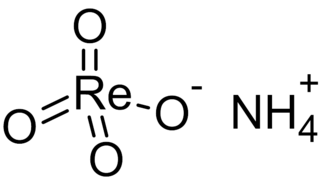
Ammonium perrhenate (APR) is the ammonium salt of perrhenic acid, NH4ReO4. It is the most common form in which rhenium is traded. It is a white salt; soluble in ethanol and water, and mildly soluble in NH4Cl. It was first described soon after the discovery of rhenium.

A double salt is a salt that contains two or more different cations or anions. Examples of double salts include alums (with the general formula MIMIII(SO4)2·12H2O) and Tutton's salts (with the general formula (MI)2MII(SO4)2·6H2O). Other examples include potassium sodium tartrate, ammonium iron(II) sulfate (Mohr's salt), potassium uranyl sulfate (used to discover radioactivity) and bromlite BaCa(CO3)2. The fluorocarbonates contain fluoride and carbonate anions. Many coordination complexes form double salts.
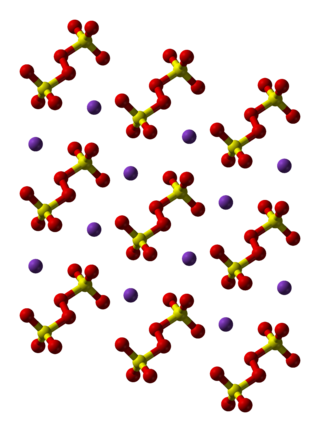
Potassium persulfate is the inorganic compound with the formula K2S2O8. Also known as potassium peroxydisulfate, it is a white solid that is sparingly soluble in cold water, but dissolves better in warm water. This salt is a powerful oxidant, commonly used to initiate polymerizations.

Sodium persulfate is the inorganic compound with the formula Na2S2O8. It is the sodium salt of peroxydisulfuric acid, H2S2O8, an oxidizing agent. It is a white solid that dissolves in water. It is almost non-hygroscopic and has good shelf-life.

The peroxydisulfate ion, S
2O2−
8, is an oxyanion, the anion of peroxydisulfuric acid. It is commonly referred to as persulfate, but this term also refers to the peroxomonosulfate ion, SO2−
5. It is also called peroxodisulfate. Approximately 500,000 tons of salts containing this anion are produced annually. Important salts include sodium persulfate (Na2S2O8), potassium persulfate (K2S2O8), and ammonium persulfate ((NH4)2S2O8). These salts are colourless, water-soluble solids that are strong oxidants.
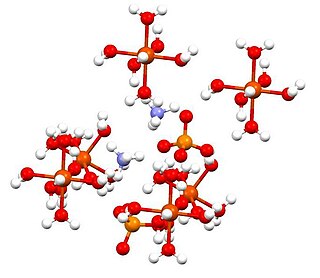
Ammonium iron(II) sulfate, or Mohr's salt, is the inorganic compound with the formula (NH4)2Fe(SO4)2(H2O)6. Containing two different cations, Fe2+ and NH+4, it is classified as a double salt of ferrous sulfate and ammonium sulfate. It is a common laboratory reagent because it is readily crystallized, and crystals resist oxidation by air. Like the other ferrous sulfate salts, ferrous ammonium sulfate dissolves in water to give the aquo complex [Fe(H2O)6]2+, which has octahedral molecular geometry. Its mineral form is mohrite.

Ammonium iron(III) sulfate, NH4Fe(SO4)2·12 H2O, or NH4[Fe(H2O)6](SO4)2·6 H2O, also known as ferric ammonium sulfate (FAS) or iron alum, is a double salt in the class of alums, which consists of compounds with the general formula AB(SO4)2 · 12 H2O. It has the appearance of weakly violet, octahedrical crystals. There has been some discussion regarding the origin of the crystals' colour, with some ascribing it to impurities in the compound, and others claiming it to be a property of the crystal itself.
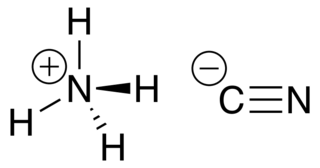
Ammonium cyanide is an unstable inorganic compound with the formula NH4CN.

Ammonium thiocyanate is an inorganic compound with the formula [NH4]+[SCN]−. It is an ammonium salt of thiocyanic acid. It consists of ammonium cations [NH4]+ and thiocyanate anions [SCN]−.
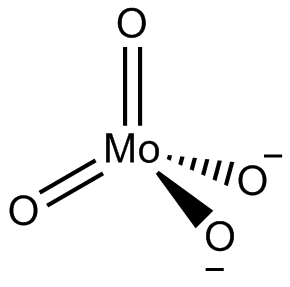
In chemistry, a molybdate is a compound containing an oxyanion with molybdenum in its highest oxidation state of 6: O−−Mo(=O)2−O−. Molybdenum can form a very large range of such oxyanions, which can be discrete structures or polymeric extended structures, although the latter are only found in the solid state. The larger oxyanions are members of group of compounds termed polyoxometalates, and because they contain only one type of metal atom are often called isopolymetalates. The discrete molybdenum oxyanions range in size from the simplest MoO2−
4, found in potassium molybdate up to extremely large structures found in isopoly-molybdenum blues that contain for example 154 Mo atoms. The behaviour of molybdenum is different from the other elements in group 6. Chromium only forms the chromates, CrO2−
4, Cr
2O2−
7, Cr
3O2−
10 and Cr
4O2−
13 ions which are all based on tetrahedral chromium. Tungsten is similar to molybdenum and forms many tungstates containing 6 coordinate tungsten.

Ammonium iodate is an inorganic salt which is sparingly soluble in cold, and moderately soluble in hot water, like all iodate salts, it is a strong oxidizer.