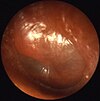
Alport syndrome is a genetic disorder affecting around 1 in 5,000-10,000 children, characterized by glomerulonephritis, end-stage kidney disease, and hearing loss. Alport syndrome can also affect the eyes, though the changes do not usually affect vision, except when changes to the lens occur in later life. Blood in urine is universal. Proteinuria is a feature as kidney disease progresses.
Renal agenesis is a medical condition in which one (unilateral) or both (bilateral) fetal kidneys fail to develop.

Pendred syndrome is a genetic disorder leading to congenital bilateral sensorineural hearing loss and goitre with euthyroid or mild hypothyroidism. There is no specific treatment, other than supportive measures for the hearing loss and thyroid hormone supplementation in case of hypothyroidism. It is named after Vaughan Pendred (1869–1946), the British doctor who first described the condition in an Irish family living in Durham in 1896. It accounts for 7.5% to 15% of all cases of congenital deafness.

Barakat syndrome is a rare disease characterized by hypoparathyroidism, sensorineural deafness and renal disease, and hence also known as HDR syndrome. It is an autosomal dominant condition with incomplete penetrance and variable expressivity that was first described by Amin J. Barakat et al. in 1977.
The metanephrogenic blastema or metanephric blastema is one of the two embryological structures that give rise to the kidney, the other being the ureteric bud.
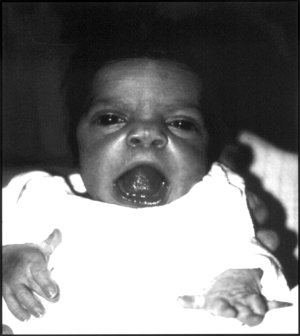
Robinow syndrome is an extremely rare genetic disorder characterized by short-limbed dwarfism, abnormalities in the head, face, and external genitalia, as well as vertebral segmentation. The disorder was first described in 1969 by human geneticist Meinhard Robinow, along with physicians Frederic N. Silverman and Hugo D. Smith, in the American Journal of Diseases of Children. By 2002, over 100 cases had been documented and introduced into medical literature.

Duane-radial ray syndrome, also known as Okihiro syndrome, is a rare autosomal dominant disorder that primarily affects the eyes and causes abnormalities of bones in the arms and hands. This disorder is considered to be a SALL4-related disorder due to the SALL4 gene mutations leading to these abnormalities. It is diagnosed by clinical findings on a physical exam as well as genetic testing and imaging. After being diagnosed, there are other evaluations that one may go through in order to determine the extent of the disease. There are various treatments for the symptoms of this disorder.
Denys–Drash syndrome (DDS) or Drash syndrome is a rare disorder or syndrome characterized by gonadal dysgenesis, nephropathy, and Wilms' tumor.

Papillorenal syndrome is an autosomal dominant genetic disorder marked by underdevelopment (hypoplasia) of the kidney and colobomas of the optic nerve.
Pendrin is an anion exchange protein that in humans is encoded by the SLC26A4 gene . Pendrin was initially identified as a sodium-independent chloride-iodide exchanger with subsequent studies showing that it also accepts formate and bicarbonate as substrates. Pendrin is similar to the Band 3 transport protein found in red blood cells. Pendrin is the protein which is mutated in Pendred syndrome, which is an autosomal recessive disorder characterized by sensorineural hearing loss, goiter and a partial organification problem detectable by a positive perchlorate test.

Townes–Brocks syndrome (TBS) is a rare genetic disease that has been described in approximately 200 cases in the published literature. It affects both males and females equally. The condition was first identified in 1972. by Philip L. Townes, who was at the time a human geneticists and Professor of Pediatrics, and Eric Brocks, who was at the time a medical student, both at the University of Rochester.

Myosin VIIA is protein that in humans is encoded by the MYO7A gene. Myosin VIIA is a member of the unconventional myosin superfamily of proteins. Myosins are actin binding molecular motors that use the enzymatic conversion of ATP - ADP + inorganic phosphate (Pi) to provide the energy for movement.

Multicystic dysplastic kidney (MCDK) is a condition that results from the malformation of the kidney during fetal development. The kidney consists of irregular cysts of varying sizes. Multicystic dysplastic kidney is a common type of renal cystic disease, and it is a cause of an abdominal mass in infants.

Eyes absent homolog 1 is a protein that in humans is encoded by the EYA1 gene.

Homeobox protein SIX1 is a protein that in humans is encoded by the SIX1 gene.

Polycystic kidney disease is a genetic disorder in which the renal tubules become structurally abnormal, resulting in the development and growth of multiple cysts within the kidney. These cysts may begin to develop in utero, in infancy, in childhood, or in adulthood. Cysts are non-functioning tubules filled with fluid pumped into them, which range in size from microscopic to enormous, crushing adjacent normal tubules and eventually rendering them non-functional as well.
Lachiewicz–Sibley syndrome is a rare autosomal dominant disorder characterized by preauricular pits and renal disease. Persons with this disease may have hypoplasic kidneys or proteinuria. This disease was first described in a Caucasian family of British and Irish descent that emigrated to Ohio in the 19th century before settling in Nebraska. Many of the members of this family still live in Nebraska, although the relatives are now scattered throughout the country.

Branchio-oculo-facial syndrome (BOFS) is a disease that arises from a mutation in the TFAP2A gene. It is a rare autosomal dominant disorder that starts to affect a child's development before birth. Symptoms of this condition include skin abnormalities on the neck, deformities of the ears and eyes, and other distinctive facial features such a cleft lip along with slow growth, mental retardation and premature graying of hair.

Otofaciocervical syndrome, also known as Fara Chlupackova syndrome, are a small group of rare developmental disorders of genetic origin which are characterized by facial dysmorphisms, long neck, preauricular and/or branchial pits, cervical muscle hypoplasia, hearing loss, and mild intellectual disabilities. Additional findings include vertebral anomalies and short stature.

Shrawan Kumar, is an Indian-American geneticist, working in the fields of molecular and population genetics. He is known for his contributions in the discovery of two genes related to Branchio-oto-renal syndrome (BOR) and Autosomal Dominant Polycystic Kidney Disease (ADPKD2).