
Barium is a chemical element; it has symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. Sulfide also refers to large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (SH−) are the conjugate acids of sulfide.

Lead(II) sulfate (PbSO4) is a white solid, which appears white in microcrystalline form. It is also known as fast white, milk white, sulfuric acid lead salt or anglesite.

Zinc sulfate describes a family of inorganic compounds with the formula ZnSO4(H2O)x. All are colorless solids. The most common form includes water of crystallization as the heptahydrate, with the formula ZnSO4·7H2O. As early as the 16th century it was prepared on the large scale, and was historically known as "white vitriol" (the name was used, for example, in 1620s by the collective writing under the pseudonym of Basil Valentine). Zinc sulfate and its hydrates are colourless solids.

Barium hydroxide is a chemical compound with the chemical formula Ba(OH)2. The monohydrate (x = 1), known as baryta or baryta-water, is one of the principal compounds of barium. This white granular monohydrate is the usual commercial form.

Zinc sulfide is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various impurities, the pure material is white, and it is widely used as a pigment. In its dense synthetic form, zinc sulfide can be transparent, and it is used as a window for visible optics and infrared optics.

Barium sulfate (or sulphate) is the inorganic compound with the chemical formula BaSO4. It is a white crystalline solid that is odorless and insoluble in water. It occurs in nature as the mineral barite, which is the main commercial source of barium and materials prepared from it. Its opaque white appearance and its high density are exploited in its main applications.

Barium chloride is an inorganic compound with the formula BaCl2. It is one of the most common water-soluble salts of barium. Like most other water-soluble barium salts, it is a white powder, highly toxic, and imparts a yellow-green coloration to a flame. It is also hygroscopic, converting to the dihydrate BaCl2·2H2O, which are colourless crystals with a bitter salty taste. It has limited use in the laboratory and industry.

Barium nitrate is the inorganic compound with the chemical formula Ba(NO3)2. It, like most barium salts, is colorless, toxic, and water-soluble. It burns with a green flame and is an oxidizer; the compound is commonly used in pyrotechnics.
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may need to be brought to this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, precipitation and other visible changes.
In chemistry, water(s) of crystallization or water(s) of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is the total mass of water in a substance at a given temperature and is mostly present in a definite (stoichiometric) ratio. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation.

Zinc selenide is the inorganic compound with the formula ZnSe. It is a lemon-yellow solid although most samples have a duller color due to the effects of oxidation. It is an intrinsic semiconductor with a band gap of about 2.70 eV at 25 °C (77 °F), equivalent to a wavelength of 459 nm. ZnSe occurs as the rare mineral stilleite, named after Hans Stille.
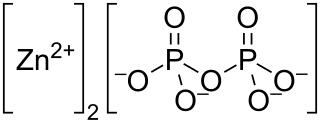
Zinc pyrophosphate (Zn2P2O7) is an ionic inorganic chemical compound composed of Zn2+ cations and pyrophosphate anions.

Barium sulfide is the inorganic compound with the formula BaS. BaS is the barium compound produced on the largest scale. It is an important precursor to other barium compounds including BaCO3 and the pigment lithopone, ZnS/BaSO4. Like other chalcogenides of the alkaline earth metals, BaS is a short wavelength emitter for electronic displays. It is colorless, although like many sulfides, it is commonly obtained in impure colored forms.

Barium peroxide is an inorganic compound with the formula BaO2. This white solid is one of the most common inorganic peroxides, and it was the first peroxide compound discovered. Being an oxidizer and giving a vivid green colour upon ignition, it finds some use in fireworks; historically, it was also used as a precursor for hydrogen peroxide.

Barium bromide is the chemical compound with the formula BaBr2. It is ionic and hygroscopic in nature.

Sachtleben Chemie is a chemicals producer, with its primary focus on the production of white pigments, fillers and extenders. The company employs some 2,200 persons and achieves annual sales of around 820 million euros (2012). Sachtleben has been a member of the multinational Venator group since 2017. The company's corporate history reaches back over 130 years. Sachtleben produces particles using titanium dioxide, zinc sulfide and barium sulfate as the chemical basis, and markets these products worldwide. The principal applications for Sachtleben products include man-made fibers, paints and other coatings, plastics, and paper. Sachtleben also supplies special particles to the foodstuffs, pharmaceuticals and cosmetics industries, and has interests in the fields of chromatography, nanotechnology, catalysis, and the production of building materials. The company leads the world in special titanium dioxide grades for printing inks and for the cosmetics, pharmaceuticals and food industries. The production facilities at all three locations apply the sophisticated sulfate-route process.
















