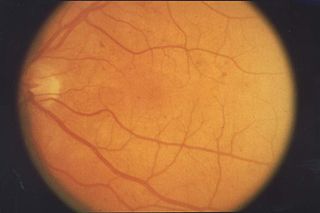
Retinopathy is any damage to the retina of the eyes, which may cause vision impairment. Retinopathy often refers to retinal vascular disease, or damage to the retina caused by abnormal blood flow. Age-related macular degeneration is technically included under the umbrella term retinopathy but is often discussed as a separate entity. Retinopathy, or retinal vascular disease, can be broadly categorized into proliferative and non-proliferative types. Frequently, retinopathy is an ocular manifestation of systemic disease as seen in diabetes or hypertension. Diabetes is the most common cause of retinopathy in the U.S. as of 2008. Diabetic retinopathy is the leading cause of blindness in working-aged people. It accounts for about 5% of blindness worldwide and is designated a priority eye disease by the World Health Organization.
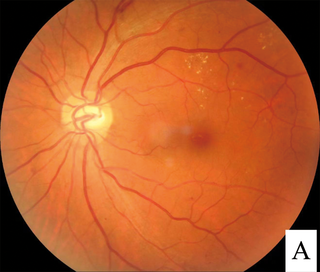
Diabetic retinopathy, is a medical condition in which damage occurs to the retina due to diabetes. It is a leading cause of blindness in developed countries.

Retinoschisis is an eye disease characterized by the abnormal splitting of the retina's neurosensory layers, usually in the outer plexiform layer. Retinoschisis can be divided into degenerative forms which are very common and almost exclusively involve the peripheral retina and hereditary forms which are rare and involve the central retina and sometimes the peripheral retina. The degenerative forms are asymptomatic and involve the peripheral retina only and do not affect the visual acuity. Some rarer forms result in a loss of vision in the corresponding visual field.

Macular edema occurs when fluid and protein deposits collect on or under the macula of the eye and causes it to thicken and swell (edema). The swelling may distort a person's central vision, because the macula holds tightly packed cones that provide sharp, clear, central vision to enable a person to see detail, form, and color that is directly in the centre of the field of view.
This is a partial list of human eye diseases and disorders.
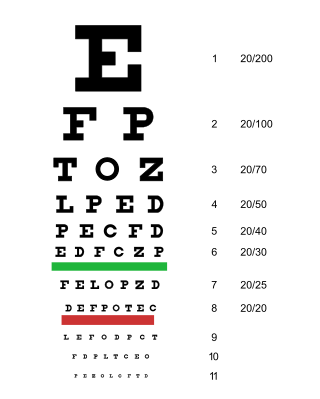
Visual or vision impairment is the partial or total inability of visual perception. In the absence of treatment such as corrective eyewear, assistive devices, and medical treatment – visual impairment may cause the individual difficulties with normal daily tasks including reading and walking. The terms low vision and blindness are often used for levels of impairment which are difficult or impossible to correct and significantly impact daily life. In addition to the various permanent conditions, fleeting temporary vision impairment, amaurosis fugax, may occur, and may indicate serious medical problems.
An eye care professional is an individual who provides a service related to the eyes or vision. It is any healthcare worker involved in eye care, from one with a small amount of post-secondary training to practitioners with a doctoral level of education.
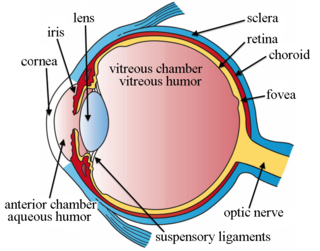
Intravitreal is a route of administration of a drug, or other substance, in which the substance is delivered into the vitreous humor of the eye. "Intravitreal" literally means "inside an eye". Intravitreal injections were first introduced in 1911 when Ohm gave an injection of air into the vitreous humor to repair a detached retina. In the mid-1940s, intravitreal injections became a standard way to administer drugs to treat endophthalmitis and cytomegalovirus retinitis.

Dilated fundus examination (DFE) is a diagnostic procedure that uses mydriatic eye drops to dilate or enlarge the pupil in order to obtain a better view of the fundus of the eye. Once the pupil is dilated, examiners use ophthalmoscopy to view the eye's interior, which makes it easier to assess the retina, optic nerve head, blood vessels, and other important features. DFE has been found to be a more effective method for evaluating eye health when compared to non-dilated examination, and is the best method of evaluating structures behind the iris. It is frequently performed by ophthalmologists and optometrists as part of an eye examination.
Ocular ischemic syndrome is the constellation of ocular signs and symptoms secondary to severe, chronic arterial hypoperfusion to the eye. Amaurosis fugax is a form of acute vision loss caused by reduced blood flow to the eye; it may be a warning sign of an impending stroke, as both stroke and retinal artery occlusion can be caused by thromboembolism due to atherosclerosis elsewhere in the body. Consequently, those with transient blurring of vision are advised to urgently seek medical attention for a thorough evaluation of the carotid artery. Anterior segment ischemic syndrome is a similar ischemic condition of anterior segment usually seen in post-surgical cases. Retinal artery occlusion leads to rapid death of retinal cells, thereby resulting in severe loss of vision.

Blurred vision is an ocular symptom where vision becomes less precise and there is added difficulty to resolve fine details.
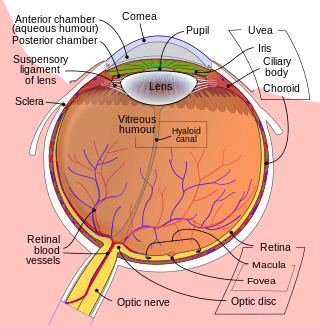
Intraocular hemorrhage is bleeding inside the eye. Bleeding can occur from any structure of the eye where there is vasculature or blood flow, including the anterior chamber, vitreous cavity, retina, choroid, suprachoroidal space, or optic disc.

A vision disorder is an impairment of the sense of vision.

Laser coagulation or laser photocoagulation surgery is used to treat a number of eye diseases and has become widely used in recent decades. During the procedure, a laser is used to finely cauterize ocular blood vessels to attempt to bring about various therapeutic benefits.
Bascom Palmer Eye Institute is the University of Miami School of Medicine's ophthalmic care, research, and education center. The institute is based in the Health District of Miami, Florida, and has been ranked consistently as the best eye hospital and vision research center in the nation.
The Vision Institute is a research center in the Quinze-Vingts National Eye Hospital in Paris, France. It is one of several such centers in Europe on eye diseases.

Irvine–Gass syndrome, pseudophakic cystoid macular edema or postcataract CME is one of the most common causes of visual loss after cataract surgery. The syndrome is named in honor of S. Rodman Irvine and J. Donald M. Gass.
Rohit Varma is an Indian-American ophthalmologist and professor of ophthalmology and preventive medicine. In 2014, he was named director of the USC Eye Institute and chairman of the Department of Ophthalmology for Keck School of Medicine of USC. In March 2016, Varma was named the interim dean of the Keck School of Medicine, and in November was named dean. In October 2017, USC announced that he stepped down as dean. In October 2018, Varma became the founding director of the Southern California Eyecare and Vision Research Institute.
Diabetic papillopathy is an ocular complication of diabetes mellitus characterized by optic disc swelling and edema of optic nerve head. The condition may affect both type 1 and type 2 diabetic patients.

Uveitic glaucoma is most commonly a progression stage of noninfectious anterior uveitis or iritis.


















