
The human immunodeficiency viruses (HIV) are two species of Lentivirus that infect humans. Over time, they cause acquired immunodeficiency syndrome (AIDS), a condition in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive. Without treatment, the average survival time after infection with HIV is estimated to be 9 to 11 years, depending on the HIV subtype.

Ascomycota is a phylum of the kingdom Fungi that, together with the Basidiomycota, forms the subkingdom Dikarya. Its members are commonly known as the sac fungi or ascomycetes. It is the largest phylum of Fungi, with over 64,000 species. The defining feature of this fungal group is the "ascus", a microscopic sexual structure in which nonmotile spores, called ascospores, are formed. However, some species of Ascomycota are asexual and thus do not form asci or ascospores. Familiar examples of sac fungi include morels, truffles, brewers' and bakers' yeast, dead man's fingers, and cup fungi. The fungal symbionts in the majority of lichens such as Cladonia belong to the Ascomycota.

Penicillium is a genus of ascomycetous fungi that is part of the mycobiome of many species and is of major importance in the natural environment, in food spoilage, and in food and drug production.

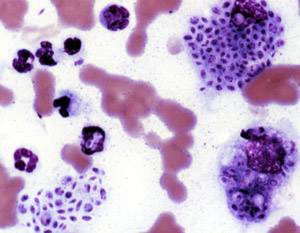
Histoplasmosis is a fungal infection caused by Histoplasma capsulatum. Symptoms of this infection vary greatly, but the disease affects primarily the lungs. Occasionally, other organs are affected; called disseminated histoplasmosis, it can be fatal if left untreated.

The bamboo rats are four species of rodents of the subfamily Rhizomyinae. They are the sole living representatives of the tribe Rhizomyini. These species are found in South Asia, Southeast Asia and East Asia.

Cryptococcus neoformans is an encapsulated yeast belonging to the class Tremellomycetes and an obligate aerobe that can live in both plants and animals. Its teleomorph is a filamentous fungus, formerly referred to Filobasidiella neoformans. In its yeast state, it is often found in bird excrement. Cryptococcus neoformans can cause disease in apparently immunocompetent, as well as immunocompromised, hosts.

Cryptococcosis is a potentially fatal fungal infection of mainly the lungs, presenting as a pneumonia, and brain, where it appears as a meningitis. Cough, difficulty breathing, chest pain and fever are seen when the lungs are infected. When the brain is infected, symptoms include headache, fever, neck pain, nausea and vomiting, light sensitivity and confusion or changes in behavior. It can also affect other parts of the body including skin, where it may appear as several fluid-filled nodules with dead tissue.

Aspergillus is a genus consisting of several hundred mold species found in various climates worldwide.
Sporotrichosis, also known as rose handler's disease, is a fungal infection that may be localised to skin, lungs, bone and joint, or become systemic. It presents with firm painless nodules that later ulcerate. Following initial exposure to Sporothrix schenckii, the disease typically progresses over a period of a week to several months. Serious complications may develop in people who have a weakened immune system.
Talaromycosis is a fungal infection that presents with painless skin lesions of the face and neck, as well as an associated fever, anaemia, and enlargement of the lymph glands and liver.

Mycoviruses, also known as mycophages, are viruses that infect fungi. The majority of mycoviruses have double-stranded RNA (dsRNA) genomes and isometric particles, but approximately 30% have positive-sense, single-stranded RNA (+ssRNA) genomes.

Sporothrix schenckii, a fungus that can be found worldwide in the environment, is named for medical student Benjamin Schenck, who in 1896 was the first to isolate it from a human specimen. The species is present in soil as well as in and on living and decomposing plant material such as peat moss. It can infect humans as well as animals and is the causative agent of sporotrichosis, commonly known as "rose handler's disease." The most common route of infection is the introduction of spores to the body through a cut or puncture wound in the skin. Infection commonly occurs in otherwise healthy individuals but is rarely life-threatening and can be treated with antifungals. In the environment it is found growing as filamentous hyphae. In host tissue it is found as a yeast. The transition between the hyphal and yeast forms is temperature dependent making S. schenckii a thermally dimorphic fungus.
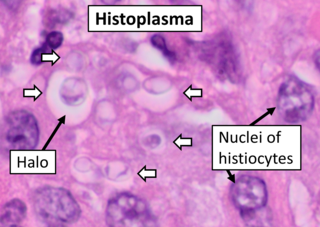
Histoplasma capsulatum is a species of dimorphic fungus. Its sexual form is called Ajellomyces capsulatus. It can cause pulmonary and disseminated histoplasmosis.

Blastomyces dermatitidis is a dimorphic fungus that causes blastomycosis, an invasive and often serious fungal infection found occasionally in humans and other animals. It lives in soil and wet, decaying wood, often in an area close to a waterway such as a lake, river or stream. Indoor growth may also occur, for example, in accumulated debris in damp sheds or shacks. The fungus is endemic to parts of eastern North America, particularly boreal northern Ontario, southeastern Manitoba, Quebec south of the St. Lawrence River, parts of the U.S. Appalachian mountains and interconnected eastern mountain chains, the west bank of Lake Michigan, the state of Wisconsin, and the entire Mississippi Valley including the valleys of some major tributaries such as the Ohio River. In addition, it occurs rarely in Africa both north and south of the Sahara Desert, as well as in the Arabian Peninsula and the Indian subcontinent. Though it has never been directly observed growing in nature, it is thought to grow there as a cottony white mold, similar to the growth seen in artificial culture at 25 °C (77 °F). In an infected human or animal, however, it converts in growth form and becomes a large-celled budding yeast. Blastomycosis is generally readily treatable with systemic antifungal drugs once it is correctly diagnosed; however, delayed diagnosis is very common except in highly endemic areas.

Dimorphic fungi are fungi that can exist in the form of both mold and yeast. This is usually brought about by change in temperature and the fungi are also described as thermally dimorphic fungi. An example is Talaromyces marneffei, a human pathogen that grows as a mold at room temperature, and as a yeast at human body temperature.
Pathogenic fungi are fungi that cause disease in humans or other organisms. Although fungi are eukaryotic, many pathogenic fungi are microorganisms. Approximately 300 fungi are known to be pathogenic to humans; their study is called "medical mycology". Fungal infections are estimated to kill more people than either tuberculosis or malaria—about two million people per year.
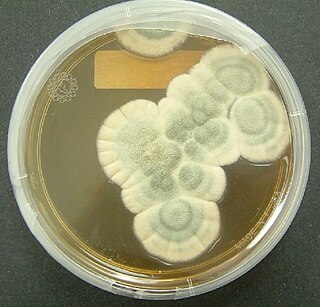
Penicillium chrysogenum is a species of fungus in the genus Penicillium. It is common in temperate and subtropical regions and can be found on salted food products, but it is mostly found in indoor environments, especially in damp or water-damaged buildings. It has been recognised as a species complex that includes P. notatum, P. meleagrinum, and P. cyaneofulvum. Molecular phylogeny has established that Alexander Fleming's first discovered penicillin producing strain is of a distinct species, P. rubens, and not of P. notatum. It has rarely been reported as a cause of human disease. It is the source of several β-lactam antibiotics, most significantly penicillin. Other secondary metabolites of P. chrysogenum include roquefortine C, meleagrin, chrysogine, 6-MSA YWA1/melanin, andrastatin A, fungisporin, secalonic acids, sorbicillin, and PR-toxin.
In biology, a pathogen, in the oldest and broadest sense, is any organism or agent that can produce disease. A pathogen may also be referred to as an infectious agent, or simply a germ.

The stages of HIV infection are acute infection, latency, and AIDS. Acute infection lasts for several weeks and may include symptoms such as fever, swollen lymph nodes, inflammation of the throat, rash, muscle pain, malaise, and mouth and esophageal sores. The latency stage involves few or no symptoms and can last anywhere from two weeks to twenty years or more, depending on the individual. AIDS, the final stage of HIV infection, is defined by low CD4+ T cell counts, various opportunistic infections, cancers, and other conditions.
Histoplasma duboisii is a saprotrophic fungus responsible for the invasive infection known as African histoplasmosis. This species is a close relative of Histoplasma capsulatum, the agent of classical histoplasmosis, and the two occur in similar habitats. Histoplasma duboisii is restricted to continental Africa and Madagascar, although scattered reports have arisen from other places usually in individuals with an African travel history. Like, H. capsulatum, H. duboisii is dimorphic – growing as a filamentous fungus at ambient temperature and a yeast at body temperature. It differs morphologically from H. capsulatum by the typical production of a large-celled yeast form. Both agents cause similar forms of disease, although H. duboisii predominantly causes cutaneous and subcutaneous disease in humans and non-human primates. The agent responds to many antifungal drug therapies used to treat serious fungal diseases.
















