3-Methylfentanyl is an opioid analgesic that is an analog of fentanyl. 3-Methylfentanyl is one of the most potent opioids, estimated to be between 400 and 6000 times stronger than morphine, depending on which isomer is used.

α-Methylacetylfentanyl is an opioid analgesic that is an analog of fentanyl. It is a Schedule I controlled substance in the United States, with a DEA ACSCN of 9815.

Parafluorofentanyl is an opioid analgesic analogue of fentanyl developed by Janssen Pharmaceuticals in the 1960s.

Betahydroxythiofentanyl (β-hydroxythiofentanyl) is an opioid analgesic that is an analog of fentanyl.

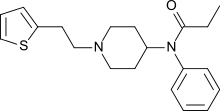
3-Methylthiofentanyl is an opioid analgesic and analogue of fentanyl.

α-Methylthiofentanyl is an opioid analgesic that is an analogue of fentanyl.

β-Hydroxyfentanyl (Fentanol) is an opioid analgesic that is an analogue of fentanyl.

Lofentanil or lofentanyl is one of the most potent opioid analgesics known and is an analogue of fentanyl, which was developed in 1960. It is most similar to the highly potent opioid carfentanil (4-carbomethoxyfentanyl), only slightly more potent. Lofentanil can be described as 3-methylcarfentanil, or 3-methyl-4-carbomethoxyfentanyl. While 3-methylfentanyl is considerably more potent than fentanyl itself, lofentanil is only slightly stronger than carfentanil. This suggests that substitution at both the 3 and 4 positions of the piperidine ring introduces steric hindrance which prevents μ-opioid affinity from increasing much further. As with other 3-substituted fentanyl derivatives such as ohmefentanyl, the stereoisomerism of lofentanil is very important, with some stereoisomers being much more potent than others.
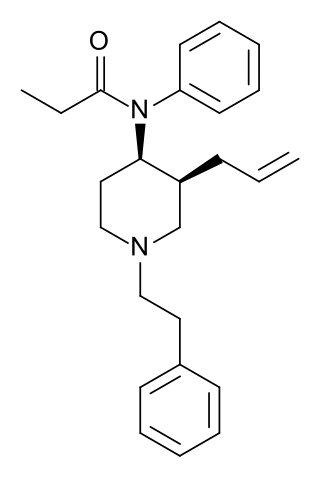
3-Allylfentanyl is an opioid analgesic that is an analogue of fentanyl.

β-Methylfentanyl is an opioid analgesic that is an analogue of fentanyl.

N-Methylnorcarfentanil (R-32395) is an opioid analgesic drug related to the highly potent animal tranquilizer carfentanil, but several thousand times weaker, being only slightly stronger than morphine. It was first synthesised by a team of chemists at Janssen Pharmaceutica led by Paul Janssen, who were investigating the structure-activity relationships of the fentanyl family of drugs. They found that replacing the phenethyl group attached to the piperidine nitrogen of fentanyl with a smaller methyl group, made it so much weaker that it was inactive as an analgesic in animals. However the same change made to the more potent analogue carfentanil retained reasonable opioid receptor activity, reflecting the higher binding affinity produced by the 4-carbomethoxy group.

R-30490 is an opioid analgesic related to the highly potent animal tranquilizer carfentanil, and with only slightly lower potency. It was first synthesised by a team of chemists at Janssen Pharmaceutica led by Paul Janssen, who were investigating the structure-activity relationships of the fentanyl family of drugs. R-30490 was found to be the most selective agonist for the μ-opioid receptor out of all the fentanyl analogues tested, but it has never been introduced for medical use in humans, although the closely related drug sufentanil is widely used for analgesia and anesthesia during major surgery.

Butyrfentanyl or butyrylfentanyl is a potent short-acting synthetic opioid analgesic drug. It is an analog of fentanyl with around one quarter of its potency. One of the first mentions of this drug can be found in document written by The College on Problem of Drug Dependence, where it is mentioned as N-butyramide fentanyl analog. This document also states that the article describing its clinical effects was published in 1987. It is an agonist for the μ-opioid receptors.

Acetylfentanyl is an opioid analgesic drug that is an analog of fentanyl. Studies have estimated acetylfentanyl to be 15 times more potent than morphine, which would mean that despite being somewhat weaker than fentanyl, it is nevertheless still several times stronger than pure heroin. It has never been licensed for medical use and instead has only been sold as a designer drug. Acetylfentanyl was discovered at the same time as fentanyl itself and had only rarely been encountered on the illicit market in the late 1980s. However, in 2013, Canadian police seized 3 kilograms of acetylfentanyl. As a μ-opioid receptor agonist, acetylfentanyl may serve as a direct substitute for heroin or other opioids. Common side effects of fentanyl analogs are similar to those of fentanyl itself, which include itching, nausea and potentially serious respiratory depression, which can be life-threatening. Fentanyl analogs have killed hundreds of people throughout Europe and the former Soviet republics since the most recent resurgence in use began in Estonia in the early 2000s, and novel derivatives continue to appear.
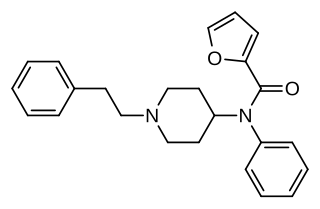
Furanylfentanyl (Fu-F) is an opioid analgesic that is an analog of fentanyl and has been sold as a designer drug. It has an ED50 value of 0.02 mg/kg in mice. This makes it approximately one fifth as potent as fentanyl.
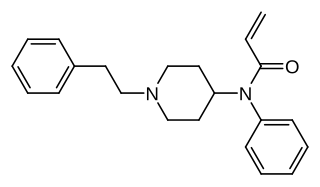
Acrylfentanyl (also known as acryloylfentanyl) is a highly potent opioid analgesic that is an analog of fentanyl and has been sold online as a designer drug. In animal studies the IC50 (the half maximal inhibitory concentration for acrylfentanyl to displace naloxone) is 1.4 nM, being slightly more potent than fentanyl itself (1.6 nM) as well as having a longer duration of action.

Methoxyacetylfentanyl, commonly known as MAF is an opioid analgesic that is an analog of fentanyl and has been sold online as a designer drug.
Tetrahydrofuranylfentanyl is an opioid analgesic that is an analog of fentanyl and has been sold online as a designer drug, first appearing in Europe in late 2016.

Orthofluorofentanyl is an opioid analgesic that is an analog of fentanyl and has been sold online as a designer drug. While the structural isomer p-fluorofentanyl was one of the first illicit fentanyl analogues identified in 1981, Orthofluorofentanyl did not appear on the illicit market until August 2016.

Isobutyrylfentanyl is an opioid analgesic that is an analog of fentanyl and has been sold online as a designer drug. It is believed to be around the same potency as butyrfentanyl but has been less widely distributed on illicit markets, though it was one of the earliest of the "new wave" of fentanyl derivatives to appear, and was reported in Europe for the first time in December 2012.