
A grape is a fruit, botanically a berry, of the deciduous woody vines of the flowering plant genus Vitis. Grapes are a non-climacteric type of fruit, generally occurring in clusters.

Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a stilbenoid, a type of natural phenol or polyphenol and a phytoalexin produced by several plants in response to injury or when the plant is under attack by pathogens, such as bacteria or fungi. Sources of resveratrol in food include the skin of grapes, blueberries, raspberries, mulberries, and peanuts.

Vitis vinifera, the common grape vine, is a species of flowering plant, native to the Mediterranean region, Central Europe, and southwestern Asia, from Morocco and Portugal north to southern Germany and east to northern Iran. As of 2012, there were between 5,000 and 10,000 varieties of Vitis vinifera grapes though only a few are of commercial significance for wine and table grape production.

Vitis rotundifolia, or muscadine, is a grapevine species native to the southeastern and south-central United States. The growth range extends from Florida to New Jersey coast, and west to eastern Texas and Oklahoma. It has been extensively cultivated since the 16th century. The plants are well-adapted to their native warm and humid climate; they need fewer chilling hours than better known varieties, and thrive in summer heat.

Phytoalexins are antimicrobial substances, some of which are antioxidative as well. They are defined not by their having any particular chemical structure or character, but by the fact that they are defensively synthesized de novo by plants that produce the compounds rapidly at sites of pathogen infection. In general phytoalexins are broad spectrum inhibitors; they are chemically diverse, and different chemical classes of compounds are characteristic of particular plant taxa. Phytoalexins tend to fall into several chemical classes, including terpenoids, glycosteroids, and alkaloids; however, the term applies to any phytochemicals that are induced by microbial infection.

Gnetum gnemon is a gymnosperm species of Gnetum, its native area spans from Mizoram and Assam in India down south through Malay Peninsula, Malay Archipelago and the Philippines in southeast Asia to the western Pacific islands. Common names include gnetum, joint fir, two leaf, melinjo, belinjo, bago, and tulip.

Stilbenoids are hydroxylated derivatives of stilbene. They have a C6–C2–C6 structure. In biochemical terms, they belong to the family of phenylpropanoids and share most of their biosynthesis pathway with chalcones. Most stilbenoids are produced by plants, and the only known exception is the antimicrobial stilbenoid drug tapinarof which is biosynthesized by the Gram-negative bacterium Photorhabdus luminescens.
The Julia olefination (also known as the Julia–Lythgoe olefination) is the chemical reaction used in organic chemistry of phenyl sulfones (1) with aldehydes (or ketones) to give alkenes (olefins)(3) after alcohol functionalization and reductive elimination using sodium amalgam or SmI2. The reaction is named after the French chemist Marc Julia.

Pterostilbene (trans-3,5-dimethoxy-4-hydroxystilbene) is a stilbenoid chemically related to resveratrol. In plants, it serves a defensive phytoalexin role.

Piceid is a stilbenoid glucoside and is a major resveratrol derivative in grape juices. It can be found in the bark of Picea sitchensis. It can also be isolated from Reynoutria japonica, the Japanese knotweed.

Piceatannol is the organic compound with the formula ( 2C6H3)2CH)2. Classified as a stilbenoid and a phenol, it is a white solid, although samples often are yellow owing to impurities.

Phenolic compounds—natural phenol and polyphenols—occur naturally in wine. These include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

ε-Viniferin is a naturally occurring phenol, belonging to the stilbenoids family. It is a resveratrol dimer.

α-Viniferin is a stilbene trimer. It can be isolated from Caragana chamlagu and from Caragana sinica and from the stem bark of Dryobalanops aromatica. It is also present in relation to resistance to Botrytis cinerea and Plasmopara viticola in Vitis vinifera and Vitis riparia. It has been shown to inhibit acetylcholinesterase.
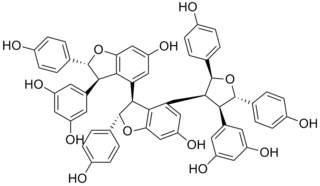
Kobophenol A is a stilbenoid. It is a tetramer of resveratrol. It can be isolated from Caragana chamlagu, from Caragana sinica and from Carex folliculata seeds.

In biochemistry, naturally occurring phenols are natural products containing at least one phenol functional group. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.

Astringin is a stilbenoid, the 3-β-D-glucoside of piceatannol. It can be found in the bark of Picea sitchensis and Picea abies.

Hopeaphenol is a stilbenoid. It is a resveratrol tetramer. It has been first isolated from Dipterocarpaceae like Shorea ovalis. It has also been isolated from wines from North Africa.

Miyabenol C is a stilbenoid. It is a resveratrol trimer. It is found in Vitis vinifera (grape), in Foeniculi fructus, in Caragana sinica.

Oligostilbenoids are oligomeric forms of stilbenoids. Some molecules are large enough to be considered polyphenols and constitute a class of tannins.

















