| |
| Names | |
|---|---|
| Preferred IUPAC name 1-Chlorobutane | |
| Other names n-Butyl chloride | |
| Identifiers | |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.361 |
| EC Number |
|
PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1127 |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C4H9Cl | |
| Molar mass | 92.57 g·mol−1 |
| Appearance | Colorless liquid [1] |
| Density | 0.89 g/mL |
| Melting point | −123.1 °C (−189.6 °F; 150.1 K) [1] |
| Boiling point | 78 °C (172 °F; 351 K) [1] |
| 0.5 g/L (20 °C) [1] | |
| Solubility | Miscible with methanol, ether[ citation needed ] |
| log P | 2.56 [2] |
| Vapor pressure | 103.4±0.1 mmHg at 25 °C [2] |
| −67.10·10−6 cm3/mol | |
Refractive index (nD) | 1.396 [2] |
| Viscosity | 0.4261 mPa·s [3] |
| Hazards | |
| GHS labelling: | |
 | |
| Danger | |
| H225 | |
| P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P370+P378, P403+P235, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | −12 °C (10 °F; 261 K) [1] |
| Safety data sheet (SDS) | Fischer MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
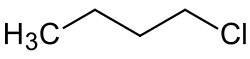
1-Chlorobutane is an alkyl halide with the chemical formula CH3(CH2)3Cl. It is a colorless, flammable liquid.