| Names | |
|---|---|
| Preferred IUPAC name 1-Iodohexane | |
| Other names 1-Hexyl iodide | |
| Identifiers | |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.010.309 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
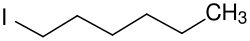
| C6H13I | |
| Molar mass | 212.074 g·mol−1 |
| Appearance | yellowish liquid |
| Density | 1.437 g/cm3 |
| Melting point | −75 °C (−103 °F; 198 K) |
| Boiling point | 181 °C (358 °F; 454 K) |
| practically insoluble | |
| Related compounds | |
Related compounds | 1-Bromohexane 1-Chlorohexane 1-Fluorohexane |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | [1] |
| GHS labelling: | |
  | |
| Danger | |
| H302, H315, H318, H319, H335 | |
| P261, P264, P264+P265, P270, P271, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P305+P354+P338, P317, P319, P321, P330, P332+P317, P337+P317, P362+P364, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
1-Iodohexane is a chemical compound from the group of aliphatic saturated halogenated hydrocarbons. The chemical formula is CH3(CH2)5I. [2] [3] It is a colorless liquid.