
Wasabi or Japanese horseradish is a plant of the family Brassicaceae, which also includes horseradish and mustard in other genera. The plant is native to Japan and the Russian Far East including Sakhalin, also the Korean Peninsula. It grows naturally along stream beds in mountain river valleys in Japan.

Horseradish is a perennial plant of the family Brassicaceae. It is a root vegetable, cultivated and used worldwide as a spice and as a condiment. The species is probably native to southeastern Europe and western Asia.

In organic chemistry, isothiocyanate is the functional group −N=C=S, formed by substituting the oxygen in the isocyanate group with a sulfur. Many natural isothiocyanates from plants are produced by enzymatic conversion of metabolites called glucosinolates. These natural isothiocyanates, such as allyl isothiocyanate, are also known as mustard oils. An artificial isothiocyanate, phenyl isothiocyanate, is used for amino acid sequencing in the Edman degradation.

Mustard oil can mean either the pressed oil used for cooking, or a pungent essential oil also known as volatile oil of mustard. The essential oil results from grinding mustard seed, mixing the grounds with water, and isolating the resulting volatile oil by distillation. It can also be produced by dry distillation of the seed. Pressed mustard oil is used as cooking oil in some cultures, but sale is restricted in some countries due to high levels of erucic acid. Varieties of mustard seed also exist that are low in erucic acid.

Allyl isothiocyanate (AITC) is a naturally occurring unsaturated isothiocyanate. The colorless oil is responsible for the pungent taste of Cruciferous vegetables such as mustard, radish, horseradish, and wasabi. This pungency and the lachrymatory effect of AITC are mediated through the TRPA1 and TRPV1 ion channels. It is slightly soluble in water, but more soluble in most organic solvents.

Fluorescein isothiocyanate (FITC) is a derivative of fluorescein used in wide-ranging applications including flow cytometry. First described in 1942, FITC is the original fluorescein molecule functionalized with an isothiocyanate reactive group (−N=C=S), replacing a hydrogen atom on the bottom ring of the structure. It is typically available as a mixture of isomers, fluorescein 5-isothiocyanate (5-FITC) and fluorescein 6-isothiocyanate (6-FITC). FITC is reactive towards nucleophiles including amine and sulfhydryl groups on proteins. It was synthesized by Robert Seiwald and Joseph Burckhalter in 1958.

Glucosinolates are natural components of many pungent plants such as mustard, cabbage, and horseradish. The pungency of those plants is due to mustard oils produced from glucosinolates when the plant material is chewed, cut, or otherwise damaged. These natural chemicals most likely contribute to plant defence against pests and diseases, and impart a characteristic bitter flavor property to cruciferous vegetables.

Phenyl isothiocyanate (PITC) is a reagent used in reversed phase HPLC. PITC is less sensitive than o-phthaldehyde (OPA) and cannot be fully automated. PITC can be used for analysing secondary amines, unlike OPA. It is also known as Edman's reagent and is used in Edman degradation.
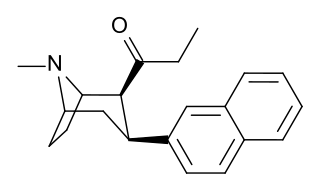
2β-Propanoyl-3β-(2-naphthyl)-tropane or WF-23 is a cocaine analogue. It is several hundred times more potent than cocaine at being a serotonin-norepinephrine-dopamine reuptake inhibitor.

4-NEMD is a potent sedative drug which acts as a selective alpha-2 adrenergic agonist. It is closely related to dexmedetomidine but is several times more potent. Like other alpha-2 agonists, it produces sedative and muscle relaxant effects but without producing respiratory depression. It is not currently used in medicine but has been researched as the basis for a potential new generation of alpha-2 agonist drugs, which may have selectivity for the different subtypes of the alpha-2 receptor. It has two isomers, with the (S) isomer being the more potent, as with medetomidine. 4-NEMD was also investigated by the United States military as an anaesthetic agent, most likely for use in surgery but possibly also for use as a non-lethal incapacitating agent, although this has not been officially confirmed.

α-Naphthylthiourea (ANTU) is an organosulfur compound with the formula C10H7NHC(S)NH2. This a white, crystalline powder although commercial samples may be off-white. It is used as a rodenticide and as such is fairly toxic. Naphthylthiourea is available as 10% active baits in suitable protein- or carbohydrate-rich materials and as a 20% tracking powder.
These drugs are known in the UK as controlled drug, because this is the term by which the act itself refers to them. In more general terms, however, many of these drugs are also controlled by the Medicines Act 1968, there are many other drugs which are controlled by the Medicines Act but not by the Misuse of Drugs Act, and some other drugs are controlled by other laws.

Naphyrone, also known as O-2482 and naphthylpyrovalerone, is a substituted cathinone drug derived from pyrovalerone that acts as a triple reuptake inhibitor, producing stimulant effects and has been reported as a novel designer drug. No safety or toxicity data is available on the drug.
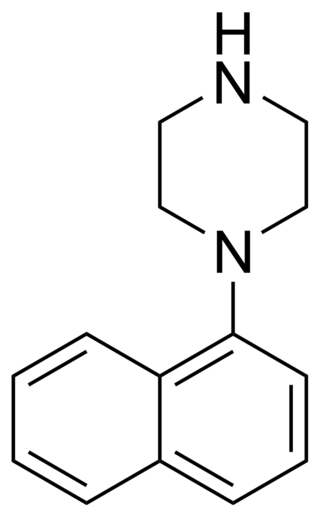
1-(1-Naphthyl)piperazine (1-NP) is a drug which is a phenylpiperazine derivative. It acts as a non-selective, mixed serotonergic agent, exerting partial agonism at the 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, and 5-HT1F receptors, while antagonizing the 5-HT2A, 5-HT2B, and 5-HT2C receptors. It has also been shown to possess high affinity for the 5-HT3, 5-HT5A, 5-HT6, and 5-HT7 receptors, and may bind to 5-HT4 and the SERT as well. In animals it produces effects including hyperphagia, hyperactivity, and anxiolysis, of which are all likely mediated predominantly or fully by blockade of the 5-HT2C receptor.
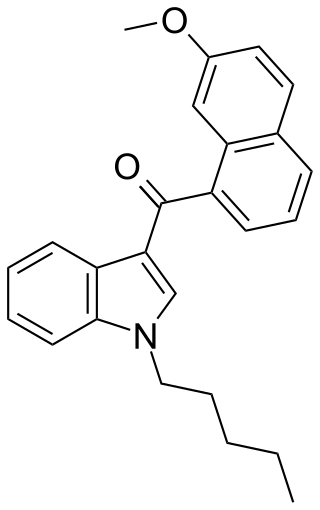
JWH-164 is a synthetic cannabinoid receptor agonist from the naphthoylindole family. It has approximately equal affinity for the CB1 and CB2 receptors, with a Ki of 6.6 nM at CB1 and 6.9 nM at CB2. JWH-164 is a positional isomer of the related compound JWH-081, but with a methoxy group at the 7-position of the naphthyl ring, rather than the 4-position as in JWH-081. Its potency is intermediate between that of JWH-081 and its ring unsubstituted derivative JWH-018, demonstrating that substitution of the naphthyl 7-position can also result in increased cannabinoid receptor binding affinity.

HU-243 (AM-4056) is a synthetic cannabinoid drug that is a single enantiomer of the hydrogenated derivative of the commonly used reference agonist HU-210. It is a methylene homologue of canbisol. It is a potent agonist at both the CB1 and CB2 receptors, with a binding affinity of 0.041 nM at the CB1 receptor, making it marginally more potent than HU-210, which had an affinity of 0.061 nM in the same assay.

Mustard is a condiment made from the seeds of a mustard plant.

Metaphit is a research chemical that acts as an acylator of NMDARAn, sigma and DAT binding sites in the CNS. It is the m-isothiocyanate derivative of phencyclidine (PCP) and binds irreversibly to the PCP binding site on the NMDA receptor complex. However, later studies suggest the functionality of metaphit is mediated by sites not involved in PCP-induced passive avoidance deficit, and not related to the NMDA receptor complex. Metaphit was also shown to prevent d-amphetamine induced hyperactivity, while significantly depleting dopamine content in the nucleus accumbens. Metaphit was the first acylating ligand used to study the cocaine receptor. It is a structural isomer of the similar research compound fourphit, as it and metaphit both are isothiocyanate substituted derivatives of an analogous scaffold shared with PCP.

Phenethyl isothiocyanate (PEITC) is a naturally occurring isothiocyanate whose precursor, gluconasturtiin is found in some cruciferous vegetables, especially watercress.

N-(1-Naphthyl)ethylenediamine is an organic compound. It is commercially available as part of Griess reagents, which find application in quantitative inorganic analysis of nitrates, nitrite and sulfonamide in blood, using the Griess test.





















