
Toluene diisocyanate (TDI) is an organic compound with the formula CH3C6H3(NCO)2. Two of the six possible isomers are commercially important: 2,4-TDI (CAS: 584-84-9) and 2,6-TDI (CAS: 91-08-7). 2,4-TDI is produced in the pure state, but TDI is often marketed as 80/20 and 65/35 mixtures of the 2,4 and 2,6 isomers respectively. It is produced on a large scale, accounting for 34.1% of the global isocyanate market in 2000, second only to MDI. Approximately 1.4 billion kilograms were produced in 2000. All isomers of TDI are colorless, although commercial samples can appear yellow.

The Knorr pyrrole synthesis is a widely used chemical reaction that synthesizes substituted pyrroles (3). The method involves the reaction of an α-amino-ketone (1) and a compound containing an electron-withdrawing group α to a carbonyl group (2).

Amitraz is a non-systemic acaricide and insecticide and has also been described as a scabicide. It was first synthesized by the Boots Co. in England in 1969. Amitraz has been found to have an insect repellent effect, works as an insecticide and also as a pesticide synergist. Its effectiveness is traced back on alpha-adrenergic agonist activity, interaction with octopamine receptors of the central nervous system and inhibition of monoamine oxidases and prostaglandin synthesis. Therefore, it leads to overexcitation and consequently paralysis and death in insects. Because amitraz is less harmful to mammals, amitraz is among many other purposes best known as insecticide against mite- or tick-infestation of dogs. It is also widely used in the beekeeping industry as a control for the Varroa destructor mite, although there are recent reports of resistance.

Phenoxy herbicides are two families of chemicals that have been developed as commercially important herbicides, widely used in agriculture. They share the part structure of phenoxyacetic acid.
Dimethoxyamphetamine (DMA) is a series of six lesser-known psychedelic drugs similar in structure to the three isomers of methoxyamphetamine and six isomers of trimethoxyamphetamine. The isomers are 2,3-DMA, 2,4-DMA, 2,5-DMA, 2,6-DMA, 3,4-DMA, and 3,5-DMA. Three of the isomers were characterized by Alexander Shulgin in his book PiHKAL. Little is known about their dangers or toxicity.

2,4 Dienoyl-CoA reductase also known as DECR1 is an enzyme which in humans is encoded by the DECR1 gene which resides on chromosome 8. This enzyme catalyzes the following reactions
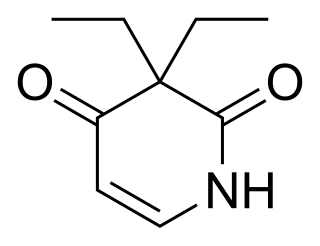
Pyrithyldione is a psychoactive drug invented in 1949. An improved method of manufacture was patented by Roche in 1959. It was used as a hypnotic or sedative and presumed to be less toxic than barbiturates. Today, this substance is no longer used. Agranulocytosis was sometimes reported as adverse effect. Pyrithyldione is also a CYP2D6 inducer but is not as potent as glutethimide. In studies, it increased the O-demethylation of codeine by 20%.
In enzymology, a 3,4-dichloroaniline N-malonyltransferase is an enzyme that catalyzes the chemical reaction
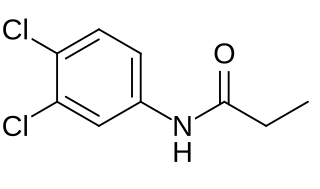
Propanil is a widely used contact herbicide. With an estimated use of about 8 million pounds in 2001, it is one of the more widely used herbicides in the United States. Propanil is said to be in use in approximately 400,000 acres of rice production each year.

Lysergic acid 2,4-dimethylazetidide (LA-SS-Az, LSZ) is an analog of LSD developed by the team led by David E. Nichols at Purdue University. It was developed as a rigid analog of LSD with the diethylamide group constrained into an azetidine ring in order to map the binding site at the 5-HT2A receptor. There are three possible stereoisomers around the azetidine ring, with the (S,S)-(+) isomer being the most active, slightly more potent than LSD itself in drug discrimination tests using trained rats.

Suritozole is an investigational cognition enhancer. It acts as a partial inverse agonist at the benzodiazepine receptor site on the GABAA ion channel complex, but does not have either anxiogenic or convulsant effects, unlike other BZD inverse agonists such as DMCM. It was investigated for the treatment of depression and Alzheimer's disease, but clinical development seems to have been discontinued.

2,4-Diaminopyrimidine is a diaminopyrimidine.

2,4-Dichlorophenoxyacetic acid is an organic compound with the chemical formula Cl2C6H3OCH2CO2H. It is usually referred to by its ISO common name 2,4-D. It is a systemic herbicide that kills most broadleaf weeds by causing uncontrolled growth, but most grasses such as cereals, lawn turf, and grassland are relatively unaffected.

Pelanserin (TR2515) is a chemical compound that acts as an antagonist of the 5-HT2 and α1-adrenergic receptors.
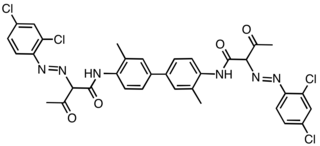
Pigment Yellow 16 is an organic compound that is classified as a diarylide pigment.
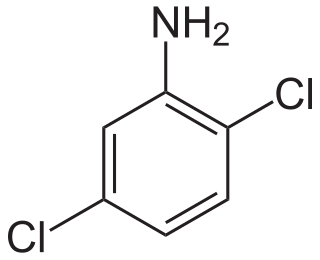
2,5-Dichloroaniline is an organic compound with the formula C6H3Cl2NH2. One of six isomers of dichloroaniline, it is a colorless solid that is insoluble in water. It is produced by hydrogenation of 1,4-dichloro-2-nitrobenzene. It is a precursor to dyes and pigments, e.g., Pigment Yellow 10.

Dichloroanilines are chemical compounds which consist of an aniline ring substituted with two chlorine atoms and have the molecular formula C6H5Cl2N. There are six isomers, varying in the positions of the chlorine atoms around the ring relative to the amino group. As aniline derivatives, they are named with the amino group in position 1. They are all colorless, although commercial samples can appear colored due to the presence of impurities. Several derivatives are used in the production of dyes and herbicides.
The molecular formula C6H5Cl2N may refer to:
3,4-Dichloroaniline is an organic compound with the formula C6H3Cl2(NH2). It is one of several isomers of dichloroaniline. It is a white solid although commercial samples often appear gray. It is a precursor to dyes, agricultural chemicals, and drugs including the antimalarial chlorproguanil and the herbicides propanil, linuron, DCMU, and diuron.

2,3-Dichloroaniline is an organic compound with the formula C6H3Cl2(NH2). It is one of several isomers of dichloroaniline. It is a colorless oil although commercial samples often appear colored. It is produced by hydrogenation of 2,3-dichloronitrobenzene.




















