Polythiophenes (PTs) are polymerized thiophenes, a sulfur heterocycle. The parent PT is an insoluble colored solid with the formula (C4H2S)n. The rings are linked through the 2- and 5-positions. Poly(alkylthiophene)s have alkyl substituents at the 3- or 4-position(s). They are also colored solids, but tend to be soluble in organic solvents.

N-Methylethanolamine is an alkanolamine with the formula CH3NHCH2CH2OH. It is flammable, corrosive, colorless, viscous liquid. It is an intermediate in the biosynthesis of choline.

Methacrylic acid, abbreviated MAA, is an organic compound with the formula CH2=C(CH3)COOH. This colorless, viscous liquid is a carboxylic acid with an acrid unpleasant odor. It is soluble in warm water and miscible with most organic solvents. Methacrylic acid is produced industrially on a large scale as a precursor to its esters, especially methyl methacrylate (MMA), and to poly(methyl methacrylate) (PMMA).

Polyphosphazenes include a wide range of hybrid inorganic-organic polymers with a number of different skeletal architectures with the backbone P-N-P-N-P-N-. In nearly all of these materials two organic side groups are attached to each phosphorus center. Linear polymers have the formula (N=PR1R2)n, where R1 and R2 are organic (see graphic). Other architectures are cyclolinear and cyclomatrix polymers in which small phosphazene rings are connected together by organic chain units. Other architectures are available, such as block copolymer, star, dendritic, or comb-type structures. More than 700 different polyphosphazenes are known, with different side groups (R) and different molecular architectures. Many of these polymers were first synthesized and studied in the research group of Harry R. Allcock.

Methyl trifluoromethanesulfonate, also commonly called methyl triflate and abbreviated MeOTf, is the organic compound with the formula CF3SO2OCH3. It is a colourless liquid which finds use in organic chemistry as a powerful methylating agent. The compound is closely related to methyl fluorosulfonate (FSO2OCH3). Although there has yet to be a reported human fatality, several cases were reported for methyl fluorosulfonate (LC50 (rat, 1 h) = 5 ppm), and methyl triflate is expected to have similar toxicity based on available evidence.

Ethyl acrylate is an organic compound with the formula CH2CHCO2CH2CH3. It is the ethyl ester of acrylic acid. It is a colourless liquid with a characteristic acrid odor. It is mainly produced for paints, textiles, and non-woven fibers. It is also a reagent in the synthesis of various pharmaceutical intermediates.

Polystannanes are organotin compounds with the formula (R2Sn)n. These polymers have been of intermittent academic interest; they are unusual because heavy elements comprise the backbone. Structurally related but better characterized (and more useful) are the polysilanes (R2Si)n.

N,N′-Methylenebisacrylamide (MBAm or MBAA, colloquially "bis") is the organic compound with the formula CH2[NHC(O)CH=CH2]2. A colorless solid, this compound is a crosslinking agent in polyacrylamides, e.g., as used for SDS-PAGE.

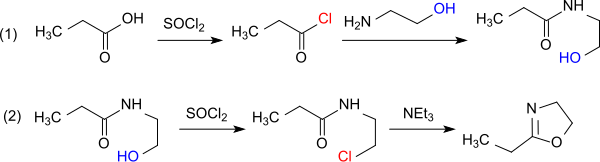
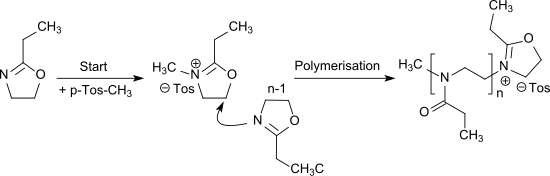
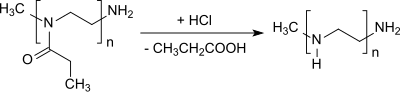
Oxazoline is a five-membered heterocyclic organic compound with the formula C3H5NO. It is the parent of a family of compounds called oxazolines, which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the unsaturated analogues of oxazolidines, and they are isomeric with isoxazolines, where the N and O are directly bonded. Two isomers of oxazoline are known, depending on the location of the double bond.
Polymers with the ability to kill or inhibit the growth of microorganisms such as bacteria, fungi, or viruses are classified as antimicrobial agents. This class of polymers consists of natural polymers with inherent antimicrobial activity and polymers modified to exhibit antimicrobial activity. Polymers are generally nonvolatile, chemically stable, and can be chemically and physically modified to display desired characteristics and antimicrobial activity. Antimicrobial polymers are a prime candidate for use in the food industry to prevent bacterial contamination and in water sanitation to inhibit the growth of microorganisms in drinking water.

The thiol-yne reaction is an organic reaction between a thiol and an alkyne. The reaction product is an alkenyl sulfide. The reaction was first reported in 1949 with thioacetic acid as reagent and rediscovered in 2009. It is used in click chemistry and in polymerization, especially with dendrimers.
Living cationic polymerization is a living polymerization technique involving cationic propagating species. It enables the synthesis of very well defined polymers and of polymers with unusual architecture such as star polymers and block copolymers and living cationic polymerization is therefore as such of commercial and academic interest.

Vinylsulfonic acid is the organosulfur compound with the chemical formula CH2=CHSO3H. It is the simplest unsaturated sulfonic acid. The C=C double bond is a site of high reactivity. Polymerization gives polyvinylsulfonic acid, especially when used as a comonomer with functionalized vinyl and (meth)acrylic acid compounds. It is a colorless, water-soluble liquid, although commercial samples can appear yellow or even red.
1-Vinylimidazole is a water-soluble basic monomer that forms quaternizable homopolymers by free-radical polymerization with a variety of vinyl and acrylic monomers. The products are functional copolymers, which are used as oil field chemicals and as cosmetic auxiliaries. 1-Vinylimidazole acts as a reactive diluent in UV lacquers, inks, and adhesives.
Dimethylaminoethyl acrylate or DMAEA is an unsaturated carboxylic acid ester having a tertiary amino group. It is a colorless to yellowish, water-miscible liquid with a pungent, amine-like odor. DMAEA is an important acrylic monomer that gives basic properties to copolymers.
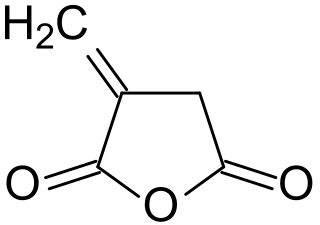
Itaconic anhydride is the cyclic anhydride of itaconic acid and is obtained by the pyrolysis of citric acid. It is a colourless, crystalline solid, which dissolves in many polar organic solvents and hydrolyzes forming itaconic acid. Itaconic anhydride and its derivative itaconic acid have been promoted as biobased "platform chemicals" and bio- building blocks.) These expectations, however, have not been fulfilled.
Polysuccinimide (PSI), also known as polyanhydroaspartic acid or polyaspartimide, is formed during the thermal polycondensation of aspartic acid and is the simplest polyimide. Polysuccinimide is insoluble in water, but soluble in some aprotic dipolar solvents. Its reactive nature makes polysuccinimide a versatile starting material for functional polymers made from renewable resources.

β-Butyrolactone is the intramolecular carboxylic acid ester (lactone) of the optically active 3-hydroxybutanoic acid. It is produced during chemical synthesis as a racemate. β-Butyrolactone is suitable as a monomer for the production of the biodegradable polyhydroxyalkanoate poly(3-hydroxybutyrate) (PHB). Polymerisation of racemic (RS)-β-butyrolactone provides (RS)-polyhydroxybutyric acid, which, however, is inferior in essential properties (e.g. strength or degradation behaviour) to the (R)-poly-3-hydroxybutyrate originating from natural sources.
Vitaliy Khutoryanskiy FRSC is a British and Kazakhstani scientist, a Professor of Formulation Science and a Royal Society Industry Fellow at the University of Reading. His research focuses on polymers, biomaterials, nanomaterials, drug delivery, and pharmaceutical sciences. Khutoryanskiy has published over 200 original research articles, book chapters, and reviews. His publications have attracted > 11000 citations and his current h-index is 50. He received several prestigious awards in recognition for his research in polymers, colloids and drug delivery as well as for contributions to research peer-review and mentoring of early career researchers. He holds several honorary professorship titles from different universities.

Hydroxyethyl acrylate is an organic chemical and a aliphatic compound. It has the formula C5H8O3 and the CAS Registry Number 818-61-1. It is REACH registered with an EU number of 212-454-9. It has dual functionality containing a polymerizable acrylic group and a terminal hydroxy group. It is used to make emulsion polymers along with other monomers and the resultant resins are used in coatings, sealants, adhesives and elastomers and other applications.