A reducing agent is an element or compound that loses an electron to an electron recipient in a redox chemical reaction.
The following outline is provided as an overview of and topical guide to organic chemistry:
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reactions, it resembles benzene. Compounds analogous to thiophene include furan (C4H4O) selenophene (C4H4Se) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring.

Polythiophenes (PTs) are polymerized thiophenes, a sulfur heterocycle. The parent PT is an insoluble colored solid with the formula (C4H2S)n. The rings are linked through the 2- and 5-positions. Poly(alkylthiophene)s have alkyl substituents at the 3- or 4-position(s). They are also colored solids, but tend to be soluble in organic solvents.
The Wolff–Kishner reduction is a reaction used in organic chemistry to convert carbonyl functionalities into methylene groups. In the context of complex molecule synthesis, it is most frequently employed to remove a carbonyl group after it has served its synthetic purpose of activating an intermediate in a preceding step. As such, there is no obvious retron for this reaction. Originally reported by Nikolai Kischner in 1911 and Ludwig Wolff in 1912, it has been applied to the total synthesis of scopadulcic acid B, aspidospermidine and dysidiolide.
Clemmensen reduction is a chemical reaction described as a reduction of ketones to alkanes using zinc amalgam and concentrated hydrochloric acid. This reaction is named after Erik Christian Clemmensen, a Danish chemist.

Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions because many reactions carry the name but do not actually involve electron transfer in the electrochemical sense of the word. Instead the relevant criterion for organic oxidation is gain of oxygen and/or loss of hydrogen, respectively.

The Wharton olefin synthesis or the Wharton reaction is a chemical reaction that involves the reduction of α,β-epoxy ketones using hydrazine to give allylic alcohols. This reaction, introduced in 1961 by P. S. Wharton, is an extension of the Wolff–Kishner reduction. The general features of this synthesis are: 1) the epoxidation of α,β-unsaturated ketones is achieved usually in basic conditions using hydrogen peroxide solution in high yield; 2) the epoxy ketone is treated with 2–3 equivalents of a hydrazine hydrate in presence of substoichiometric amounts of acetic acid. This reaction occurs rapidly at room temperature with the evolution of nitrogen and the formation of an allylic alcohol. It can be used to synthesize carenol compounds. Wharton's initial procedure has been improved.
Catalyst poisoning refers to the partial or total deactivation of a catalyst by a chemical compound. Poisoning refers specifically to chemical deactivation, rather than other mechanisms of catalyst degradation such as thermal decomposition or physical damage. Although usually undesirable, poisoning may be helpful when it results in improved catalyst selectivity.
Deoxygenation is a chemical reaction involving the removal of oxygen atoms from a molecule. The term also refers to the removal molecular oxygen (O2) from gases and solvents, a step in air-free technique and gas purifiers. As applied to organic compounds, deoxygenation is a component of fuels production as well a type of reaction employed in organic synthesis, e.g. of pharmaceuticals.
In enzymology, a thiophene-2-carbonyl-CoA monooxygenase (EC 1.14.99.35) is an enzyme that catalyzes the chemical reaction
Ludwig Wolff, born in Neustadt in Palatinate, was a German chemist.
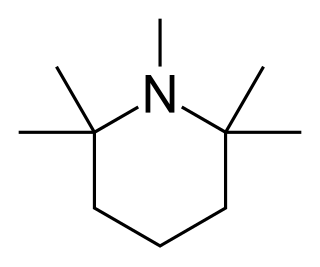
Pempidine is a ganglion-blocking drug, first reported in 1958 by two research groups working independently, and introduced as an oral treatment for hypertension.
Huang Minlon, Huang-Minlon, or Huang Minglong was a Chinese organic chemist and pharmaceutical scientist. Huang is considered a pioneer and founder of modern pharmaceutical industries in China.

In organic chemistry, carbonyl reduction is the organic reduction of any carbonyl group by a reducing agent.

Docosanedioic acid is a dicarboxylic acid with the linear formula HOOC(CH
2)
20COOH.
The Mozingo reduction, also known as Mozingo reaction or thioketal reduction, is a chemical reaction capable of fully reducing a ketone or aldehyde to the corresponding alkane. The reaction scheme is as follows:

Allylcyclopentane is a hydrocarbon that has the formula C8H14. This compound is a colourless liquid at room temperature. It has been prepared from cyclopentanemagnesium bromide and allyl bromide.
The molecular formula C5H6S may refer to:

Nikolai Matveyevich Kischner was a Russian chemist and member of the Russian Academy of Sciences.










