
An organic sulfide or thioether is a functional group in organosulfur chemistry with the connectivity C–S–C as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A sulfide is similar to an ether except that it contains a sulfur atom in place of the oxygen. The grouping of oxygen and sulfur in the periodic table suggests that the chemical properties of ethers and sulfides are somewhat similar, though the extent to which this is true in practice varies depending on the application.

In chemistry thioesters are compounds with the functional group R–S–CO–R'. They are analogous to carboxylate esters with the sulfur in the thioester playing the role of the linking oxygen in the carboxylate ester. They are the product of esterification between a carboxylic acid and a thiol. In biochemistry, the best-known thioesters are derivatives of coenzyme A, e.g., acetyl-CoA.
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reactions, it resembles benzene. Compounds analogous to thiophene include furan (C4H4O) selenophene (C4H4Se) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring.

Polythiophenes (PTs) are polymerized thiophenes, a sulfur heterocycle. The parent PT is an insoluble colored solid with the formula (C4H2S)n. The rings are linked through the 2- and 5-positions. Poly(alkylthiophene)s have alkyl substituents at the 3- or 4-position(s). They are also colored solids, but tend to be soluble in organic solvents.

Tienilic acid or ticrynafen (USAN) is a loop diuretic drug with uric acid-lowering (uricosuric) action, formerly marketed for the treatment of hypertension. It was approved by FDA on May 2, 1979, and withdrawn in 1982, after case reports in the United States indicated a link between the use of ticrynafen and hepatitis.
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is essential for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.
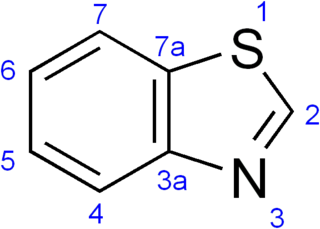
Benzothiazole is an aromatic heterocyclic compound with the chemical formula C
7H
5NS. It is colorless, slightly viscous liquid. Although the parent compound, benzothiazole is not widely used, many of its derivatives are found in commercial products or in nature. Firefly luciferin can be considered a derivative of benzothiazole.

Benzothiophene is an aromatic organic compound with a molecular formula C8H6S and an odor similar to naphthalene (mothballs). It occurs naturally as a constituent of petroleum-related deposits such as lignite tar. Benzothiophene has no household use. In addition to benzo[b]thiophene, a second isomer is known: benzo[c]thiophene.
Tetrahydrothiophene is an organosulfur compound with the formula (CH2)4S. It contains a five-membered ring consisting of four carbon atoms and a sulfur atom. It is the saturated analog of thiophene. It is a volatile, colorless liquid with an intensely unpleasant odor. It is also known as thiophane, thiolane, or THT.

Terthiophene is the organic compound with the formula [C4H3S]2C4H2S. It is an oligomer of the heterocycle thiophene, a shorter oligomer is dithienyl, and the parent polymer is polythiophene. In the most common isomer of terthiophene, two thienyl groups are connected via their 2 positions to a central thiophene, also at the carbon atoms flanking the sulfur.

Dibenzothiophene (DBT) is the organosulfur compound consisting of two benzene rings fused to a central thiophene ring. It is a colourless solid that is chemically somewhat similar to anthracene. This tricyclic heterocycle, and especially its alkyl substituted derivatives, occur widely in heavier fractions of petroleum.

The Sommelet reaction is an organic reaction in which a benzyl halide is converted to an aldehyde by action of hexamine and water.
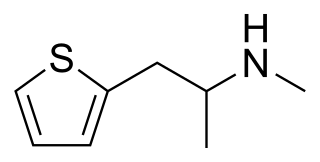
Methiopropamine (MPA) is a thiophene ring-based structural analog of methamphetamine originally reported in 1942. Chemically it is not a phenethylamine or amphetamine and is not their functional analog either. It originally appeared for public sale in the UK in December 2010 as a "research chemical" or "legal high", recently branded as Blow. It has limited popularity as a recreational stimulant.
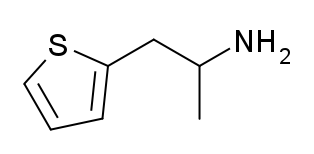
Thiopropamine is a stimulant drug which is an analogue of amphetamine where the phenyl ring has been replaced by thiophene. It has similar stimulant effects to amphetamine but with around one third the potency. The N-methyl and thiophen-3-yl analogues are also known and are somewhat more potent, though still generally weaker than the corresponding amphetamines.
3-Bromothiophene is an organosulfur compound with the formula C4H3BrS. It is a colorless liquid. It is a precursor to the antibiotic timentin and the vasodilator cetiedil.
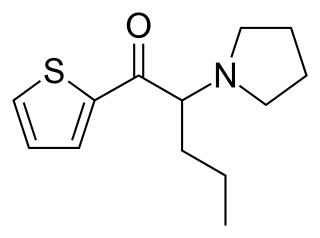
α-Pyrrolidinopentiothiophenone is a synthetic stimulant of the cathinone class that has been sold online as a designer drug. It is an analogue of α-PVP where the phenyl ring has been replaced by thiophene.

Trifluoroperacetic acid is an organofluorine compound, the peroxy acid analog of trifluoroacetic acid, with the condensed structural formula CF
3COOOH. It is a strong oxidizing agent for organic oxidation reactions, such as in Baeyer–Villiger oxidations of ketones. It is the most reactive of the organic peroxy acids, allowing it to successfully oxidise relatively unreactive alkenes to epoxides where other peroxy acids are ineffective. It can also oxidise the chalcogens in some functional groups, such as by transforming selenoethers to selones. It is a potentially explosive material and is not commercially available, but it can be quickly prepared as needed. Its use as a laboratory reagent was pioneered and developed by William D. Emmons.

Thienothiophene usually refers to any of three structurally related derivatives of thiophene with the formula C6H4S2. In order of importance, they are: thieno(3,2-b)thiophene, thieno(2,3-b)thiophene, and thieno(3,4-b)thiophene. Other isomers feature S(IV) and are less stable. Thieno[2,3-b]thiophene was the first member of the series to be isolated. It was obtained in very low yield upon heating citric acid, a source of a six-carbon linear chain, with P4S10. More efficient syntheses of this and the other two stable thienothiophenes involve cyclization reactions of substituted thiophenes.
3,4-Ethylenedioxythiophene (EDOT) is the organosulfur compound with the formula C2H4O2C4H2S. The molecule consists of thiophene, substituted at the 3 and 4 positions with an ethylene glycolyl unit. It is a colorless viscous liquid.
2-Bromothiophene is an organosulfur compound with the formula C4H3BrS. It is a colorless liquid. Unlike 3-bromothiophene, the 2-bromo isomer is prepared directly by partial bromination of thiophene. It is a precursor to several drugs, including tipepidine, ticlopidine, and clopidogrel.















