In biochemistry, a ligase is an enzyme that can catalyze the joining (ligation) of two molecules by forming a new chemical bond. This is typically via hydrolysis of a small pendant chemical group on one of the molecules, typically resulting in the formation of new C-O, C-S, or C-N bonds. For example, DNA ligase can join two complementary fragments of nucleic acid by forming phosphodiester bonds, and repair single stranded breaks that arise in double stranded DNA during replication.

Glutamine is an α-amino acid that is used in the biosynthesis of proteins. Its side chain is similar to that of glutamic acid, except the carboxylic acid group is replaced by an amide. It is classified as a charge-neutral, polar amino acid. It is non-essential and conditionally essential in humans, meaning the body can usually synthesize sufficient amounts of it, but in some instances of stress, the body's demand for glutamine increases, and glutamine must be obtained from the diet. It is encoded by the codons CAA and CAG. It is named after glutamic acid, which in turn is named after its discovery in cereal proteins, gluten.
Pyruvic acid (IUPAC name: 2-oxopropanoic acid, also called acetoic acid) (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.
Carbonation is the chemical reaction of carbon dioxide to give carbonates, bicarbonates, and carbonic acid. In chemistry, the term is sometimes used in place of carboxylation, which refers to the formation of carboxylic acids.
The Kolbe–Schmitt reaction or Kolbe process is a carboxylation chemical reaction that proceeds by treating phenol with sodium hydroxide to form sodium phenoxide, then heating sodium phenoxide with carbon dioxide under pressure, then treating the product with sulfuric acid. The final product is an aromatic hydroxy acid which is also known as salicylic acid.
Oxidative decarboxylation is a decarboxylation reaction caused by oxidation. Most are accompanied by α- Ketoglutarate α- Decarboxylation caused by dehydrogenation of hydroxyl carboxylic acids such as carbonyl carboxylic acid, malic acid, isocitric acid, etc.
Carboxylation is a chemical reaction in which a carboxylic acid is produced by treating a substrate with carbon dioxide. The opposite reaction is decarboxylation. In chemistry, the term carbonation is sometimes used synonymously with carboxylation, especially when applied to the reaction of carbanionic reagents with CO2. More generally, carbonation usually describes the production of carbonates.

Hydroxynaphthol blue is an azo dye. It is used for determining the endpoint in complexometric titrations/Metal Titration.

Pamoic acid, also called embonic acid, is a 2-Naphthoic acid derivative. Salts and esters of pamoic acid are known as pamoates or embonates. It can be prepared by the reaction of 3-hydroxy-2-naphthoic acid with formaldehyde.
The molecular formula C11H8O2 (molar mass: 172.18 g/mol, exact mass: 172.0524 u) may refer to:
Metal carbon dioxide complexes are coordination complexes that contain carbon dioxide ligands. Aside from the fundamental interest in the coordination chemistry of simple molecules, studies in this field are motivated by the possibility that transition metals might catalyze useful transformations of CO2. This research is relevant both to organic synthesis and to the production of "solar fuels" that would avoid the use of petroleum-based fuels.
Naphthoyl (naphthalenecarbonyl) is an acyl group derived from naphthoic acid.
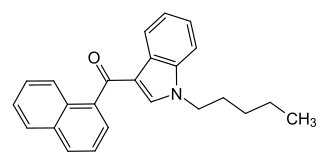
Naphthoylindoles are a class of synthetic cannabinoids.
1-hydroxy-2-naphthoate hydroxylase (EC 1.14.13.135, 1-hydroxy-2-naphthoic acid hydroxylase) is an enzyme with systematic name 1-hydroxy-2-naphthoate,NAD(P)H:oxygen oxidoreductase (2-hydroxylating, decarboxylating). This enzyme catalyses the following chemical reaction
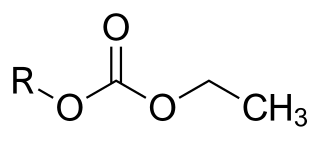
Etabonate or ethyl carbonate is the chemical group with formula –CO
3–C
2H
5, or H
3C–CH
2–O–C(=O)–O–. The names are also used for esters R–OCO
2C
2H
5, for the anion [C
2H
5OCO−
2], and for salts of the latter.

1-Bromonaphthalene is an organic compound with the formula C10H7Br.

3-Hydroxy-2-naphthoic acid is an organic compound with the formula C10H6(OH)(CO2H). It is one of the several hydroxynaphthoic acids. It is a precursor to some azo dyes and pigments. It is prepared by carboxylation of 2-naphthol by the Kolbe–Schmitt reaction.

1-Naphthoic acid is an organic compound with the formula C10H7CO2H. It is one of two isomeric monocarboxylic acids of naphthalene, the other one being 2-naphthoic acid. In general the hydroxynaphthoic acids are more widly use than the parent naphthoic acids.
Naphthoic acid, also known as Naphthalenecarboxylic acid may refer to:
2-Hydroxy-1-naphthoic acid is an organic compound with the formula C10H6(OH)(CO2H). It is prepared by carboxylation of 2-naphthol by the Kolbe–Schmitt reaction. It is one of several hydroxynaphthoic acids.









