In molecular biology, biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism.

Homoserine (also called isothreonine) is an α-amino acid with the chemical formula HO2CCH(NH2)CH2CH2OH. L-Homoserine is not one of the common amino acids encoded by DNA. It differs from the proteinogenic amino acid serine by insertion of an additional -CH2- unit into the backbone. Homoserine, or its lactone form, is the product of a cyanogen bromide cleavage of a peptide by degradation of methionine.

Amino acid synthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can synthesize 11 of the 20 standard amino acids. These 11 are called the non-essential amino acids).

In enzymology, an aspartate-semialdehyde dehydrogenase is an enzyme that is very important in the biosynthesis of amino acids in prokaryotes, fungi, and some higher plants. It forms an early branch point in the metabolic pathway forming lysine, methionine, leucine and isoleucine from aspartate. This pathway also produces diaminopimelate which plays an essential role in bacterial cell wall formation. There is particular interest in ASADH as disabling this enzyme proves fatal to the organism giving rise to the possibility of a new class of antibiotics, fungicides, and herbicides aimed at inhibiting it.

In enzymology, a phosphoribosylanthranilate isomerase (PRAI) is an enzyme that catalyzes the third step of the synthesis of the amino acid tryptophan.

In molecular biology, the protein domain SAICAR synthase is an enzyme which catalyses a reaction to create SAICAR. In enzymology, this enzyme is also known as phosphoribosylaminoimidazolesuccinocarboxamide synthase. It is an enzyme that catalyzes the chemical reaction

The enzyme 3-dehydroquinate synthase catalyzes the chemical reaction
The Aminoshikimate pathway is a biochemical pathway present in some plants, which has been studied by biologists, biochemists and especially those interested in manufacture of novel antibiotic drugs. The pathway is a novel variation of the shikimate pathway. The aminoshikimate pathway was first discovered and studied in the rifamycin B producer Amycolatopsis mediterranei. Its end product, 3-amino-5-hydroxybenzoate, serves as an initiator for polyketide synthases in the biosynthesis of ansamycins.
The shikimate pathway is a seven-step metabolic pathway used by bacteria, archaea, fungi, algae, some protozoans, and plants for the biosynthesis of folates and aromatic amino acids. This pathway is not found in animal cells.

3-Deoxy-D-arabinoheptulosonate 7-phosphate (DAHP) synthase is the first enzyme in a series of metabolic reactions known as the shikimate pathway, which is responsible for the biosynthesis of the amino acids phenylalanine, tyrosine, and tryptophan. Since it is the first enzyme in the shikimate pathway, it controls the amount of carbon entering the pathway. Enzyme inhibition is the primary method of regulating the amount of carbon entering the pathway. Forms of this enzyme differ between organisms, but can be considered DAHP synthase based upon the reaction that is catalyzed by this enzyme.
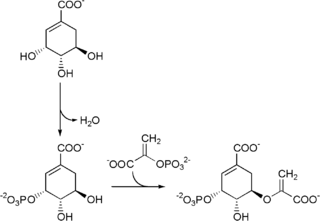
5-enolpyruvylshikimate-3-phosphate (EPSP) synthase is an enzyme produced by plants and microorganisms. EPSPS catalyzes the chemical reaction:

3-Deoxy-D-arabino-heptulosonic acid 7-phosphate (DAHP) is a 7-carbon ulonic acid. This compound is found in the shikimic acid biosynthesis pathway and is an intermediate in the production of aromatic amino acids.
UDP-glucuronic acid dehydrogenase (UDP-4-keto-hexauronic acid decarboxylating) (EC 1.1.1.305, UDP-GlcUA decarboxylase, ArnADH) is an enzyme with systematic name UDP-glucuronate:NAD+ oxidoreductase (decarboxylating). This enzyme catalyses the following chemical reaction
3-dehydroquinate synthase II (EC 1.4.1.24, DHQ synthase II, MJ1249 (gene), aroB' (gene)) is an enzyme with systematic name 2-amino-3,7-dideoxy-D-threo-hept-6-ulosonate:NAD+ oxidoreductase (deaminating). This enzyme catalyses the following chemical reaction
6-deoxy-5-ketofructose 1-phosphate synthase is an enzyme with systematic name 2-oxopropanal:D-fructose 1,6-bisphosphate glycerone-phosphotransferase. This enzyme catalyses the following chemical reaction
UDP-4-amino-4,6-dideoxy-N-acetyl-alpha-D-glucosamine transaminase is an enzyme with systematic name UDP-4-amino-4,6-dideoxy-N-acetyl-alpha-D-glucosamine:2-oxoglutarate aminotransferase. This enzyme catalyses the following chemical reaction
UDP-2-acetamido-2-deoxy-ribo-hexuluronate aminotransferase is an enzyme with systematic name UDP-2-acetamido-3-amino-2,3-dideoxy-alpha-D-glucuronate:2-oxoglutarate aminotransferase. This enzyme catalyses the following chemical reaction

4-Hydroxy-tetrahydrodipicolinate synthase (EC 4.3.3.7, dihydrodipicolinate synthase, dihydropicolinate synthetase, dihydrodipicolinic acid synthase, L-aspartate-4-semialdehyde hydro-lyase (adding pyruvate and cyclizing), dapA (gene)) is an enzyme with the systematic name L-aspartate-4-semialdehyde hydro-lyase (adding pyruvate and cyclizing; (4S)-4-hydroxy-2,3,4,5-tetrahydro-(2S)-dipicolinate-forming). This enzyme catalyses the following chemical reaction

L-Aspartic-4-semialdehyde is an α-amino acid derivative of aspartate. It is an important intermediate in the aspartate pathway, which is a metabolic pathway present in bacteria and plants. The aspartate pathway leads to the biosynthesis of a variety of amino acids from aspartate, including lysine, methionine, and threonine.












