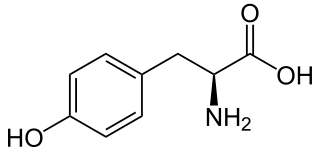
L-Tyrosine or tyrosine or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Greek tyrós, meaning cheese, as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is encoded by the codons UAC and UAU in messenger RNA.

A catecholamine is a monoamine neurotransmitter, an organic compound that has a catechol and a side-chain amine.
In chemistry, hydroxylation can refer to:
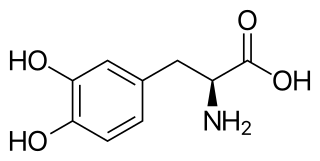
l-DOPA, also known as levodopa and l-3,4-dihydroxyphenylalanine, is an amino acid that is made and used as part of the normal biology of some plants and animals, including humans. Humans, as well as a portion of the other animals that utilize l-DOPA, make it via biosynthesis from the amino acid l-tyrosine. l-DOPA is the precursor to the neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), which are collectively known as catecholamines. Furthermore, l-DOPA itself mediates neurotrophic factor release by the brain and CNS. l-DOPA can be manufactured and in its pure form is sold as a psychoactive drug with the INN levodopa; trade names include Sinemet, Pharmacopa, Atamet, and Stalevo. As a drug, it is used in the clinical treatment of Parkinson's disease and dopamine-responsive dystonia.

Caffeic acid is an organic compound that is classified as a hydroxycinnamic acid. This yellow solid consists of both phenolic and acrylic functional groups. It is found in all plants because it is an intermediate in the biosynthesis of lignin, one of the principal components of woody plant biomass and its residues.

d-DOPA is similar to l-DOPA (levodopa), but with opposite chirality. Levo- and dextro- rotation refer to a molecule's ability to rotate planes of polarized light in one or the other direction. Whereas l-DOPA is moderately effective in the treatment of Parkinson's disease (PD) and dopamine-responsive dystonia (DRD) by stimulating the production of dopamine in the brain, d-DOPA is biologically inactive.
Tyrosine hydroxylase or tyrosine 3-monooxygenase is the enzyme responsible for catalyzing the conversion of the amino acid L-tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA). It does so using molecular oxygen (O2), as well as iron (Fe2+) and tetrahydrobiopterin as cofactors. L-DOPA is a precursor for dopamine, which, in turn, is a precursor for the important neurotransmitters norepinephrine (noradrenaline) and epinephrine (adrenaline). Tyrosine hydroxylase catalyzes the rate limiting step in this synthesis of catecholamines. In humans, tyrosine hydroxylase is encoded by the TH gene, and the enzyme is present in the central nervous system (CNS), peripheral sympathetic neurons and the adrenal medulla. Tyrosine hydroxylase, phenylalanine hydroxylase and tryptophan hydroxylase together make up the family of aromatic amino acid hydroxylases (AAAHs).

Phenanthrenoids are chemical compounds formed with a phenanthrene backbone. These compounds occur naturally in plants, although they can also be synthesized.
Dihydroxyphenylalanine may refer to either of two chemical compounds:
In enzymology, a stizolobate synthase (EC 1.13.11.29) is an enzyme that catalyzes the chemical reaction
In enzymology, a stizolobinate synthase (EC 1.13.11.30) is an enzyme that catalyzes the chemical reaction
In enzymology, a dihydroxyphenylalanine ammonia-lyase (EC 4.3.1.11, entry deleted) is a non-existing enzyme that catalyzes the chemical reaction
The enzyme phenylalanine ammonia lyase (EC 4.3.1.24) catalyzes the conversion of L-phenylalanine to ammonia and trans-cinnamic acid.:
In enzymology, a dihydroxyphenylalanine transaminase is an enzyme that catalyzes the chemical reaction

α-Difluoromethyl-3,4-dihydroxyphenylalanine is a DOPA decarboxylase inhibitor.
The biosynthesis of phenylpropanoids involves a number of enzymes.
The TH gene codes for the enzyme tyrosine hydroxylase.
Phenylalanine N-monooxygenase (EC 1.14.14.40, phenylalanine N-hydroxylase, CYP79A2) is an enzyme with systematic name L-phenylalanine,NADPH:oxygen oxidoreductase (N-hydroxylating). This enzyme catalyses the following chemical reaction
The enzyme 3,4-dihydroxyphenylalanine reductive deaminase (EC 4.3.1.22, reductive deaminase, DOPA-reductive deaminase, DOPARDA; systematic name 3,4-dihydroxy-L-phenylalanine ammonia-lyase (3,4-dihydroxyphenylpropanoate-forming)) catalyses the following chemical reaction
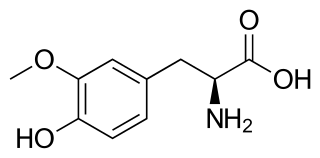
3-O-Methyldopa (3-OMD) is one of the most important metabolites of L-DOPA, a drug used in the treatment of the Parkinson's disease.










