
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The word "aromatic" originates from the past grouping of molecules based on smell, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation with their smell.
In chemistry, a structural isomer of a compound is another compound whose molecule has the same number of atoms of each element, but with logically distinct bonds between them. The term metamer was formerly used for the same concept.
para-Cresol, also 4-methylphenol, is an organic compound with the formula CH3C6H4(OH). It is a colourless solid that is widely used intermediate in the production of other chemicals. It is a derivative of phenol and is an isomer of o-cresol and m-cresol.
meta-Cresol, also 3-methylphenol, is an organic compound with the formula CH3C6H4(OH). It is a colourless, viscous liquid that is used as an intermediate in the production of other chemicals. It is a derivative of phenol and is an isomer of p-cresol and o-cresol.
A complexometric indicator is an ionochromic dye that undergoes a definite color change in presence of specific metal ions. It forms a weak complex with the ions present in the solution, which has a significantly different color from the form existing outside the complex. Complexometric indicators are also known as pM indicators.
Xylenols are organic compounds with the formula (CH3)2C6H3OH. They are volatile colorless solids or oily liquids. They are derivatives of phenol with two methyl groups at various positions relative to the hydroxyl group. Six isomers exist, of which 2,6-xylenol with both methyl groups in an ortho position with respect to the hydroxyl group is the most important. The name xylenol is a portmanteau of the words xylene and phenol.

2,6-Xylenol is a chemical compound which is one of the six isomers of xylenol. It is also commonly known as 2,6-dimethylphenol (DMP). It is a colorless solid.
Chloroxylenol, also known as para-chloro-meta-xylenol (PCMX), is an antiseptic and disinfectant which is used for skin disinfection, and together with alcohol for cleaning surgical instruments. It is also used within a number of household disinfectants and wound cleaners. It is thought to act by disrupting microbial cell walls and inactivating cellular enzymes, and is less effective than some other available agents. It is available as a liquid.

2,4-Dimethyl-6-tert-butylphenol is the organic compound with the formula Me2(tert-Bu)C6H2OH (Me = methyl, tert-Bu = tertiary butyl). It is a colorless oil that is classified as an alkylated phenol.
Ethylphenol (4-EP) is an organic compound with the formula C2H5C6H4OH. It is one of three isomeric ethylphenols. A white solid, it occurs as an impurity in xylenols and as such is used in the production of some commercial phenolic resins. It is also a precursor to 4-vinylphenol.

2,6-Xylidine is an organic compound with the formula C6H3(CH3)2NH2. It is one of several isomeric xylidines. It is a colorless viscous liquid. Commercially significant derivatives are the anesthetics lidocaine, bupivacaine, mepivacaine, and etidocaine.
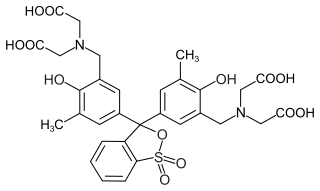
Xylenol orange is an organic reagent, most commonly used as a tetrasodium salt as an indicator for metal titrations. When used for metal titrations, it will appear red in the titrand and become yellow once it reaches its endpoint. Historically, commercial preparations of it have been notoriously impure, sometimes consisting of as little as 20% xylenol orange, and containing large amounts of semi-xylenol orange and iminodiacetic acid. Purities as high as 90% are now available.

Coalite is a brand of low-temperature coke used as a smokeless fuel. The title refers to the residue left behind when coal is carbonised at 640 °C (1,184 °F). It was invented by Thomas Parker in 1904. In 1936 the Smoke Abatement Society awarded its inventor a posthumous gold medal.

Alkylphenols are a family of organic compounds obtained by the alkylation of phenols. The term is usually reserved for commercially important propylphenol, butylphenol, amylphenol, heptylphenol, octylphenol, nonylphenol, dodecylphenol and related "long chain alkylphenols" (LCAPs). Methylphenols and ethylphenols are also alkylphenols, but they are more commonly referred to by their specific names, cresols and xylenols.

Methiocarb is a carbamate pesticide which is used as an insecticide, bird repellent, acaricide and molluscicide since the 1960s. Methiocarb has contact and stomach action on mites and neurotoxic effects on molluscs. Seeds treated with methiocarb also affect birds. Other names for methiocarb are mesurol and mercaptodimethur.

Iminodiacetic acid is the organic compound with the formula HN(CH2CO2H)2, often abbreviated to IDA. A white solid, the compound is a dicarboxylic acid amine (the nitrogen atom forms a secondary amino group, not an imino group as the name suggests). The iminodiacetate dianion is a tridentate ligand, forming metal complexes by forming two, fused, five membered chelate rings. The proton on the nitrogen atom can be replaced by a carbon atom of a polymer to create an ion-exchange resin, such as chelex 100. Complexes of IDA and EDTA were introduced in the early 1950's by Schwarzenbach.
The molecular formula C8H10O may refer to:
ortho-Cresol (IUPAC name: 2-methylphenol, also known as 2-hydroxytoluene or ortho-Toluenol) is an organic compound with the formula CH3C6H4(OH). It is a colourless solid that is widely used intermediate in the production of other chemicals. It is a derivative of phenol and is an isomer of p-cresol and m-cresol.

3-Ethylphenol is an organic compound with the formula C2H5C6H4OH. It is one of three isomeric ethylphenols. A colorless liquid, it occurs as an impurity in xylenols and as such is used in the production of commercial phenolic resins.
2-Ethylphenol is an organic compound with the formula C2H5C6H4OH. It is one of three isomeric ethylphenols. A colorless liquid, it occurs as an impurity in xylenols and as such is used in the production of commercial phenolic resins. It is produced by ethylation of phenol using ethylene or ethanol in the presence of aluminium phenolate.


















