
Butanone, also known as methyl ethyl ketone (MEK) or ethyl methyl ketone, is an organic compound with the formula CH3C(O)CH2CH3. This colorless liquid ketone has a sharp, sweet odor reminiscent of acetone. It is produced industrially on a large scale, but occurs in nature only in trace amounts. It is partially soluble in water, and is commonly used as an industrial solvent. It is an isomer of another solvent, tetrahydrofuran.
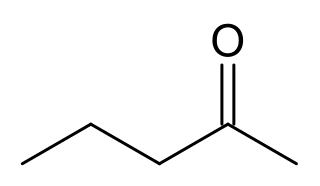
2-Pentanone or methyl propyl ketone (MPK) is a ketone and solvent of minor importance. It is comparable to methyl ethyl ketone, but has a lower solvency and is more expensive. It occurs naturally in Nicotiana tabacum (Tobacco) and blue cheese as a metabolic product of Penicillium mold growth.

3-Pentanone is a simple, symmetrical dialkyl ketone. It is a colorless liquid ketone with an odor like that of acetone. It is soluble in about 25 parts water, but miscible with organic solvents.
Pentanone may refer to the following ketones containing five carbon atoms:
Perfluoro(2-methyl-3-pentanone) is a fluorinated ketone with the structural formula CF3CF2C(=O)CF(CF3)2, a fully-fluorinated analog of ethyl isopropyl ketone. It is used as an electronics coolant liquid and fire protection fluid sold commercially by 3M under brand names such as Novec 1230, Novec 649, and FK-5-1-12. It is also known as “waterless water” or “dry water”.

Ethyl isopropyl ketone (2-methyl-pentan-3-one) is an aliphatic ketone with used as a reagent in organic chemistry and as a solvent.
The Corey–Kim oxidation is an oxidation reaction used to synthesize aldehydes and ketones from primary and secondary alcohols. It is named for American chemist and Nobel Laureate Elias James Corey and Korean-American chemist Choung Un Kim.
![<span class="mw-page-title-main">Eschenmoser's salt</span> Ionic compound with the formula [(H3C–)2N–CH2]I](https://upload.wikimedia.org/wikipedia/commons/thumb/2/28/Eschenmosersalz.png/320px-Eschenmosersalz.png)
In organic chemistry, Eschenmoser's salt is the ionic, organic compound [(CH3)2NCH2]I. It is the iodide salt of the dimethylaminomethylene cation [(CH3)2NCH2]+.
The molecular formula C5H10O may refer to:
The molecular formula C6H12O may refer to:
Heptanone may refer to the following ketones with seven carbon atoms the formula C7H14O:
Hexanone may refer to the following ketones containing six carbon atoms:

3-Hexanone (ethyl propyl ketone) is an organic compound with the formula C6H12O. It is a ketone used as a solvent and as a chemical intermediate.
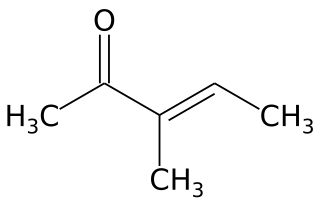
3-Methyl-3-penten-2-one is an unsaturated aliphatic ketone. It is an isomer of mesityl oxide and isomesityl oxide. It is a precursor of 3-methyl-2-pentanone and is obtained by acid-catalyzed dehydration of 4-hydroxy-3-methyl-2-pentanone. It is used as an intermediate in organic chemistry syntheses.

α-Pyrrolidinohexiophenone is a synthetic stimulant drug of the cathinone class developed in the 1960s which has been reported as a novel designer drug.

4-Methylpentedrone, is a stimulant drug of the cathinone class that has been sold online as a designer drug. It is a higher homolog of 4-methylmethcathinone (mephedrone) and 4-methylbuphedrone (4-MeMABP), and the p-methyl derivative of pentedrone. It can also be viewed as the methylamino analog of pyrovalerone.
The K & L Avenue Landfill, also known by the spelling K&L Avenue Landfill, is an 87-acre (35-hectare) Superfund site accessed from KL Avenue in Oshtemo Township, Kalamazoo County, Michigan. It is one of six Superfund sites in the Kalamazoo River watershed.

4-Methyl-α-ethylaminopentiophenone (4-MEAP) is a designer drug of the cathinone class. It is a higher homolog of 4-methylpentedrone (4-MPD) with an ethyl group in place of the methyl group. 4-MEAP has been found in samples of drugs sold as 4-MPD.
Hydroxymethylation is a chemical reaction that installs the CH2OH group. The transformation can be implemented in many ways and applies to both industrial and biochemical processes.







![<span class="mw-page-title-main">Eschenmoser's salt</span> Ionic compound with the formula [(H3C–)2N–CH2]I](https://upload.wikimedia.org/wikipedia/commons/thumb/2/28/Eschenmosersalz.png/320px-Eschenmosersalz.png)




