
Bromine is a chemical element; it has symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig and Antoine Jérôme Balard, its name was derived from Ancient Greek βρῶμος (bromos) 'stench', referring to its sharp and pungent smell.

N,N-Dimethylaniline (DMA) is an organic chemical compound, a substituted derivative of aniline. It is a tertiary amine, featuring a dimethylamino group attached to a phenyl group. This oily liquid is colourless when pure, but commercial samples are often yellow. It is an important precursor to dyes such as crystal violet.

Aniline is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans.
In organic chemistry, an aryl halide is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. Haloarenes are different from haloalkanes because they exhibit many differences in methods of preparation and properties. The most important members are the aryl chlorides, but the class of compounds is so broad that there are many derivatives and applications.
Werner Emmanuel Bachmann was an American chemist. Bachmann was born in Detroit, Michigan where he studied chemistry and chemical engineering at Wayne State University and later at the University of Michigan in Ann Arbor nearby. He completed his doctorate under Moses Gomberg and spent the rest of his academic career at the University of Michigan.

Hydrogen bromide is the inorganic compound with the formula HBr. It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room temperature. Aqueous solutions that are 47.6% HBr by mass form a constant-boiling azeotrope mixture that boils at 124.3 °C (255.7 °F). Boiling less concentrated solutions releases H2O until the constant-boiling mixture composition is reached.
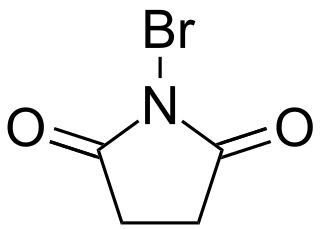
N-Bromosuccinimide or NBS is a chemical reagent used in radical substitution, electrophilic addition, and electrophilic substitution reactions in organic chemistry. NBS can be a convenient source of Br•, the bromine radical.

Moses Gomberg was a chemistry professor at the University of Michigan. He was elected to the National Academy of Sciences and the American Philosophical Society, and served as president of the American Chemical Society.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. The parent compound where R is hydrogen, is diazenylium.

Tetraphenylmethane is an organic compound consisting of a methane core with four phenyl substituents. It was first synthesized by Moses Gomberg in 1898.
The Gomberg–Bachmann reaction, named for the Russian-American chemist Moses Gomberg and the American chemist Werner Emmanuel Bachmann, is an aryl-aryl coupling reaction via a diazonium salt.
The Béchamp reduction is a chemical reaction that converts aromatic nitro compounds to their corresponding anilines using iron as the reductant:

Thiocyanogen, (SCN)2, is a pseudohalogen derived from the pseudohalide thiocyanate, [SCN]−, with behavior intermediate between dibromine and diiodine. This hexatomic compound exhibits C2 point group symmetry and has the connectivity NCS-SCN.

Benzenediazonium tetrafluoroborate is an organic compound with the formula [C6H5N2]BF4. It is a salt of a diazonium cation and tetrafluoroborate. It exists as a colourless solid that is soluble in polar solvents. It is the parent member of the aryldiazonium compounds, which are widely used in organic chemistry.

Bromine monofluoride is a quite unstable interhalogen compound with the chemical formula BrF. It can be produced through the reaction of bromine trifluoride (or bromine pentafluoride) and bromine. Due to its lability, the compound can be detected but not isolated:

Propargyl bromide, also known as 3-bromo-prop-1-yne, is an organic compound with the chemical formula HC≡CCH2Br. A colorless liquid, it is a halogenated organic compound consisting of propyne with a bromine substituent on the methyl group. It has a lachrymatory effect, like related compounds. The compound is used as a reagent in organic synthesis.
The Pschorr cyclization is a name reaction in organic chemistry, which was named after its discoverer, the German chemist Robert Pschorr (1868-1930). It describes the intramolecular substitution of aromatic compounds via aryldiazonium salts as intermediates and is catalyzed by copper. The reaction is a variant of the Gomberg-Bachmann reaction. The following reaction scheme shows the Pschorr cyclization for the example of phenanthrene:

Solvent Yellow 7 is an aromatic organic molecule and a common azo dye with a formula of C6H5N2C6H4OH. It has a phenolic hydroxyl and an azo group in the same molecule.

Cyanuric bromide is a heterocyclic compound with formula C3N3Br3. It contains a six-membered ring of alternating nitrogen and carbon atoms, with a bromine atom attached to each carbon. It is formed by the spontaneous trimerisation of cyanogen bromide.



















