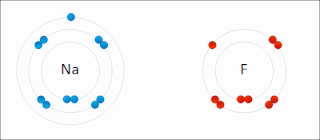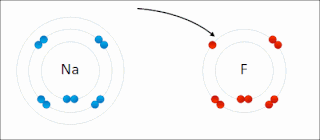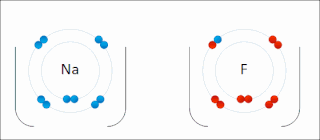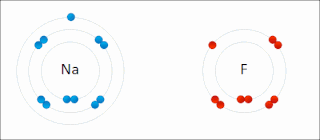
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in ionic compounds. It is one of the main types of bonding along with covalent bonding and metallic bonding. Ions are atoms with an electrostatic charge. Atoms that gain electrons make negatively charged ions. Atoms that lose electrons make positively charged ions. This transfer of electrons is known as electrovalence in contrast to covalence. In the simplest case, the cation is a metal atom and the anion is a nonmetal atom, but these ions can be of a more complex nature, e.g. molecular ions like NH+
4 or SO2−
4. In simpler words, an ionic bond results from the transfer of electrons from a metal to a non-metal in order to obtain a full valence shell for both atoms.
A chemical species is a chemical substance or ensemble composed of chemically identical molecular entities that can explore the same set of molecular energy levels on a characteristic or delineated time scale. These energy levels determine the way the chemical species will interact with others. The species can be atom, molecule, ion, radical, and it has a chemical name and chemical formula. The term is also applied to a set of chemically identical atomic or molecular structural units in a solid array.
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small relative to the energy parameters of the component. While traditional chemistry concentrates on the covalent bond, supramolecular chemistry examines the weaker and reversible non-covalent interactions between molecules. These forces include hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi–pi interactions and electrostatic effects.

The term supermolecule was introduced by Karl Lothar Wolf et al. (Übermoleküle) in 1937 to describe hydrogen-bonded acetic acid dimers. The study of non-covalent association of complexes of molecules has since developed into the field of supramolecular chemistry. The term supermolecule is sometimes used to describe supramolecular assemblies, which are complexes of two or more molecules that are not covalently bonded. The term supermolecule is also used in biochemistry to describe complexes of biomolecules, such as peptides and oligonucleotides composed of multiple strands.
A supramolecular assembly is a complex of molecules held together by noncovalent bonds. While a supramolecular assembly can be simply composed of two molecules, or a defined number of stoichiometrically interacting molecules within a quaternary complex, it is more often used to denote larger complexes composed of indefinite numbers of molecules that form sphere-, rod-, or sheet-like species. Colloids, liquid crystals, biomolecular condensates, micelles, liposomes and biological membranes are examples of supramolecular assemblies. The dimensions of supramolecular assemblies can range from nanometers to micrometers. Thus they allow access to nanoscale objects using a bottom-up approach in far fewer steps than a single molecule of similar dimensions.
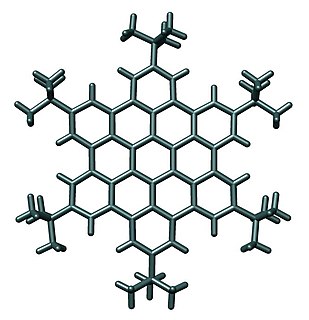
Supramolecular electronics is the experimental field of supramolecular chemistry that bridges the gap between molecular electronics and bulk plastics in the construction of electronic circuitry at the nanoscale 1. In supramolecular electronics, assemblies of pi-conjugated systems on the 5 to 100 nanometer length scale are prepared by molecular self-assembly with the aim to fit these structures between electrodes. With single-molecules as researched in molecular electronics at the 5 nanometer scale this would be impractical. Nanofibers can be prepared from polymers such as polyaniline and polyacetylene 12. Chiral oligo(p-phenylenevinylene)s self-assemble in a controlled fashion into (helical) wires 3. An example of actively researched compounds in this field are certain coronenes.
Dynamic covalent chemistry (DCvC) is a synthetic strategy employed by chemists to make complex supramolecular assemblies from discrete molecular building blocks. DCvC has allowed access to complex assemblies such as covalent organic frameworks, molecular knots, polymers, and novel macrocycles. Not to be confused with dynamic combinatorial chemistry, DCvC concerns only covalent bonding interactions. As such, it only encompasses a subset of supramolecular chemistries.

In supramolecular chemistry, molecular encapsulation is the confinement of a guest molecule inside the cavity of a supramolecular host molecule. Examples of supramolecular host molecule include carcerands and endohedral fullerenes.

In chemistry, a salt bridge is a combination of two non-covalent interactions: hydrogen bonding and ionic bonding. Ion pairing is one of the most important noncovalent forces in chemistry, in biological systems, in different materials and in many applications such as ion pair chromatography. It is a most commonly observed contribution to the stability to the entropically unfavorable folded conformation of proteins. Although non-covalent interactions are known to be relatively weak interactions, small stabilizing interactions can add up to make an important contribution to the overall stability of a conformer. Not only are salt bridges found in proteins, but they can also be found in supramolecular chemistry. The thermodynamics of each are explored through experimental procedures to access the free energy contribution of the salt bridge to the overall free energy of the state.
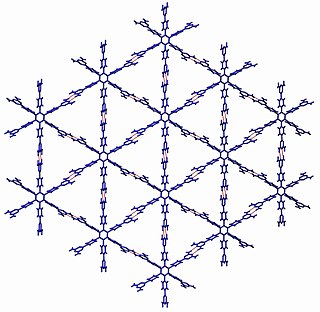
Crystal engineering is the design and synthesis of molecular solid state structures with desired properties, based on an understanding and use of intermolecular interactions. The two main strategies currently in use for crystal engineering are based on hydrogen bonding and coordination bonding. These may be understood with key concepts such as the supramolecular synthon and the secondary building unit.
The term ‘polymer’ refers to large molecules whose structure is composed of multiple repeating units and the prefix ‘supra’ meaning ‘beyond the limits of’. Supramolecular polymers are a new category of polymers that can potentially be used for material applications beyond the limits of conventional polymers. By definition, supramolecular polymers are polymeric arrays of monomeric units that are connected by reversible and highly directional secondary interactions–that is, non-covalent bonds. These non-covalent interactions include van der Waals interactions, hydrogen bonding, Coulomb or ionic interactions, π-π stacking, metal coordination, halogen bonding, chalcogen bonding, and host–guest interaction. The direction and strength of the interactions are precisely tuned so that the array of molecules behaves as a polymer in dilute and concentrated solution, as well as in the bulk.
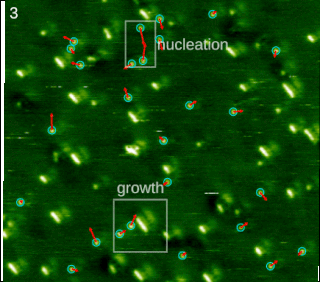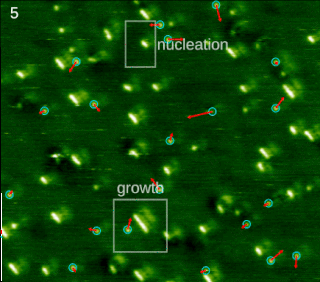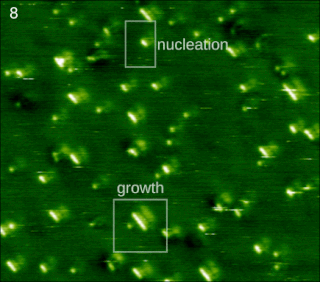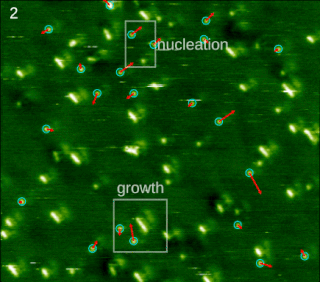
Molecular self-assembly is the process by which molecules adopt a defined arrangement without guidance or management from an outside source. There are two types of self-assembly. These are intramolecular self-assembly and intermolecular self-assembly. Commonly, the term molecular self-assembly refers to intermolecular self-assembly, while the intramolecular analog is more commonly called folding.
In chemistry, the term supramolecular chirality is used to describe supramolecular assemblies that are non-superposable on their mirror images.
Harry Laurence Anderson is a British chemist in the Department of Chemistry, University of Oxford. He is well known for his contributions in the syntheses of supramolecular systems, exploration of the extraordinary physical properties of large pi-conjugated systems, and synthesis of cyclo[18]carbon. He is a Professor of Chemistry at Keble College, Oxford.

Supramolecular catalysis is not a well-defined field but it generally refers to an application of supramolecular chemistry, especially molecular recognition and guest binding, toward catalysis. This field was originally inspired by enzymatic system which, unlike classical organic chemistry reactions, utilizes non-covalent interactions such as hydrogen bonding, cation-pi interaction, and hydrophobic forces to dramatically accelerate rate of reaction and/or allow highly selective reactions to occur. Because enzymes are structurally complex and difficult to modify, supramolecular catalysts offer a simpler model for studying factors involved in catalytic efficiency of the enzyme. Another goal that motivates this field is the development of efficient and practical catalysts that may or may not have an enzyme equivalent in nature.
Pierangelo Metrangolo is vicepres of IUPAC and an Italian chemist with interests in supramolecular chemistry and functional materials. He also has an interest in crystal engineering, in particular by using the halogen bond.

Philip Alan Gale is a British chemist and Head of the School of Chemistry at the University of Sydney. He is notable for his work on the supramolecular chemistry of anions.
The Supramolecular Chemistry Award was a prestigious award that was made by the Royal Society of Chemistry for studies leading to the design of functionally useful supramolecular species. The first award was made in 2001 and the final award in 2020. It was awarded biennially.

Subi Jacob George is an Indian organic chemist, known for his work in the fields of supramolecular chemistry, materials chemistry and polymer chemistry. His research interests includes organic and supramolecular synthesis, functional organic materials, supramolecular polymers, chiral amplification, and hybrid materials.

Roeland J. M. Nolte is a Dutch chemist, known for his work in the fields of organic chemistry, biochemistry, polymer chemistry, and supramolecular chemistry. He is an emeritus Royal Netherlands of Arts and Sciences professor and an emeritus professor of Organic Chemistry at Radboud University in Nijmegen, The Netherlands. Currently, he holds a special chair, i.e. professor of Molecular Nanotechnology, at this university. Nolte is considered to be one of the pioneers of the field of supramolecular chemistry, which encompasses the design and synthesis of new chemical structures from low molecular weight compounds and biopolymers using so-called non-covalent interactions. He published many studies on supramolecular assembly and biomimetic catalysts, which find applications in the field of nanomaterials and medicine.












