
In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. These compounds contain a distinctive functional group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

In organic chemistry, an oxime is an organic compound belonging to the imines, with the general formula RR’C=N−OH, where R is an organic side-chain and R' may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides with general structure R1C(=NOH)NR2R3.

An organic acid anhydride is an acid anhydride that is also an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the parent acid is a carboxylic acid, the formula of the anhydride being (RC(O))2O. Symmetrical acid anhydrides of this type are named by replacing the word acid in the name of the parent carboxylic acid by the word anhydride. Thus, (CH3CO)2O is called acetic anhydride.Mixed (or unsymmetrical) acid anhydrides, such as acetic formic anhydride (see below), are known, whereby reaction occurs between two different carboxylic acids. Nomenclature of unsymmetrical acid anhydrides list the names of both of the reacted carboxylic acids before the word "anhydride" (for example, the dehydration reaction between benzoic acid and propanoic acid would yield "benzoic propanoic anhydride").
In organic chemistry, the Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl functional group by formaldehyde and a primary or secondary amine or ammonia. The final product is a β-amino-carbonyl compound also known as a Mannich base. Reactions between aldimines and α-methylene carbonyls are also considered Mannich reactions because these imines form between amines and aldehydes. The reaction is named after Carl Mannich.

Phosphorous acid is the compound described by the formula H3PO3. This acid is diprotic, not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. Organic derivatives of phosphorous acid, compounds with the formula RPO3H2, are called phosphonic acids.

Gold(III) chloride, traditionally called auric chloride, is an inorganic compound of gold and chlorine with the molecular formula Au2Cl6. The "III" in the name indicates that the gold has an oxidation state of +3, typical for many gold compounds. It has two forms, the monohydrate (AuCl3·H2O) and the anhydrous form, which are both hygroscopic and light-sensitive solids. This compound is a dimer of AuCl3. This compound has a few uses, such as an oxidizing agent and for catalyzing various organic reactions.
The organic compound ethyl acetoacetate (EAA) is the ethyl ester of acetoacetic acid. It is a colorless liquid. It is widely used as a chemical intermediate in the production of a wide variety of compounds.

In organic chemistry, phosphonates or phosphonic acids are organophosphorus compounds containing C−PO(OR)2 groups, where R is an organic group. If R is hydrogen then the compound is a dialkyl phosphite, which is a different functional group. Phosphonic acids, typically handled as salts, are generally nonvolatile solids that are poorly soluble in organic solvents, but soluble in water and common alcohols.

The Petasis reaction is the multi-component reaction of an amine, a carbonyl, and a vinyl- or aryl-boronic acid to form substituted amines.

Triflic acid, the short name for trifluoromethanesulfonic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest known acids. Triflic acid is mainly used in research as a catalyst for esterification. It is a hygroscopic, colorless, slightly viscous liquid and is soluble in polar solvents.

Methyl vinyl ketone (MVK, IUPAC name: butenone) is the organic compound with the formula CH3C(O)CH=CH2. It is a reactive compound classified as an enone, in fact the simplest example thereof. It is a colorless, flammable, highly toxic liquid with a pungent odor. It is soluble in water and polar organic solvents. It is a useful intermediate in the synthesis of other compounds.

In organic chemistry, hydroxamic acids are a class of organic compounds having a general formula R−C(=O)−N(−OH)−R' bearing the functional group −C(=O)−N(−OH)−, where R and R' are typically organyl groups or hydrogen. They are amides wherein the nitrogen atom has a hydroxyl substituent. They are often used as metal chelators.
Trimethylsilanol (TMS) is an organosilicon compound with the formula (CH3)3SiOH. The Si centre bears three methyl groups and one hydroxyl group. It is a colourless volatile liquid.

EDDHA or ethylenediamine-N,N′-bis(2-hydroxyphenylacetic acid) is a chelating agent. Like EDTA, it binds metal ions as a hexadentate ligand, using two amines, two phenolate centers, and two carboxylates as the six binding sites. The complexes are typically anionic. The ligand itself is a white, water-soluble powder. Both the free ligand and its tetraanionic chelating agent are abbreviated EDDHA. In contrast to EDDHA, most related aminopolycarboxylic acid chelating agents feature tertiary amines and few have phenolate groups.

EDTMP or ethylenediamine tetra(methylene phosphonic acid) is a phosphonic acid. It has chelating and anti corrosion properties. EDTMP is the phosphonate analog of EDTA. It is classified as a nitrogenous organic polyphosphonic acid.

In organic chemistry, carbonyl reduction is the conversion of any carbonyl group, usually to an alcohol. It is a common transformation that is practiced in many ways. Ketones, aldehydes, carboxylic acids, esters, amides, and acid halides - some of the most pervasive functional groups, -comprise carbonyl compounds. Carboxylic acids, esters, and acid halides can be reduced to either aldehydes or a step further to primary alcohols, depending on the strength of the reducing agent. Aldehydes and ketones can be reduced respectively to primary and secondary alcohols. In deoxygenation, the alcohol group can be further reduced and removed altogether by replacement with H.
In organic chemistry, Lewis acid catalysis is the use of metal-based Lewis acids as catalysts for organic reactions. The acids act as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early and late d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen, nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.
Aminophosphonates are organophosphorus compounds with the formula (RO)2P(O)CH2NR2. These compounds are structural analogues of amino acids in which a carboxylic moiety is replaced by phosphonic acid or related groups. Acting as antagonists of amino acids, they inhibit enzymes involved in amino acid metabolism and thus affect the physiological activity of the cell. These effects may be exerted as antibacterial, plant growth regulatory or neuromodulatory. They can act as ligands, and heavy metal complexes with aminophosphonates have medical applications.
Transition metal amino acid complexes are a large family of coordination complexes containing the conjugate bases of the amino acids, the 2-aminocarboxylates. Amino acids are prevalent in nature, and all of them function as ligands toward the transition metals. Not included in this article are complexes of the amides and ester derivatives of amino acids. Also excluded are the polyamino acids including the chelating agents EDTA and NTA.
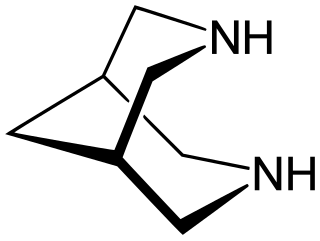
Bispidine (3,7-diazabicyclo[3.3.1]nonane) is an organic compound that is classified as a bicyclic diamine. Although synthetic, it is related structurally to natural alkaloid sparteine. It is a white crystalline solid. It has been widely investigated as a chelating agent. Many derivatives are known.















