
An antacid is a substance which neutralizes stomach acidity and is used to relieve heartburn, indigestion or an upset stomach. Some antacids have been used in the treatment of constipation and diarrhea. Marketed antacids contain salts of aluminium, calcium, magnesium, or sodium. Some preparations contain a combination of two salts, such as magnesium carbonate and aluminium hydroxide.

Camazepam is a benzodiazepine psychoactive drug, marketed under the brand names Albego, Limpidon and Paxor. It is the dimethyl carbamate ester of temazepam, a metabolite of diazepam. While it possesses anxiolytic, anticonvulsant, skeletal muscle relaxant and hypnotic properties it differs from other benzodiazepines in that its anxiolytic properties are particularly prominent but has comparatively limited anticonvulsant, hypnotic and skeletal muscle relaxant properties.

Ethyl loflazepate is a drug which is a benzodiazepine derivative. It possesses anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties. In animal studies it was found to have low toxicity, although in rats evidence of pulmonary phospholipidosis occurred with pulmonary foam cells developing with long-term use of very high doses. Its elimination half-life is 51–103 hours. Its mechanism of action is similar to other benzodiazepines. Ethyl loflazepate also produces an active metabolite which is stronger than the parent compound. Ethyl loflazepate was designed to be a prodrug for descarboxyloflazepate, its active metabolite. It is the active metabolite which is responsible for most of the pharmacological effects rather than ethyl loflazepate. The main metabolites of ethyl loflazepate are descarbethoxyloflazepate, loflazepate and 3-hydroxydescarbethoxyloflazepate. Accumulation of the active metabolites of ethyl loflazepate are not affected by those with kidney failure or impairment. The symptoms of an overdose of ethyl loflazepate include sleepiness, agitation and ataxia. Hypotonia may also occur in severe cases. These symptoms occur much more frequently and severely in children. Death from therapeutic maintenance doses of ethyl loflazepate taken for 2 – 3 weeks has been reported in 3 elderly patients. The cause of death was asphyxia due to benzodiazepine toxicity. High doses of the antidepressant fluvoxamine may potentiate the adverse effects of ethyl loflazepate.
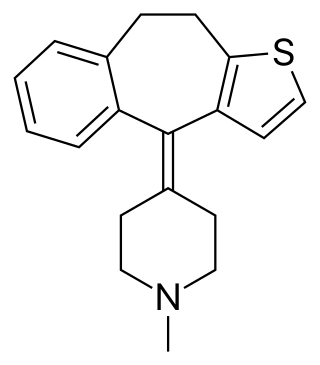
Pizotifen (INN) or pizotyline (USAN), trade name Sandomigran, is a benzocycloheptene-based drug used as a medicine, primarily as a preventive to reduce the frequency of recurrent migraine headaches.

Fluspirilene is a diphenylbutylpiperidine typical antipsychotic drug, used for the treatment of schizophrenia. It is administered intramuscularly. It was discovered at Janssen Pharmaceutica in 1963. A 2007 systematic review investigated the efficacy of fluspirilene decanoate for people with schizophrenia:
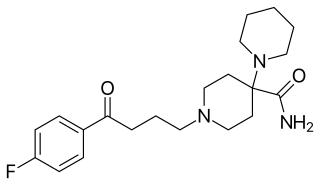
Pipamperone, also known as carpiperone and floropipamide or fluoropipamide, and as floropipamide hydrochloride (JAN), is a typical antipsychotic of the butyrophenone family used in the treatment of schizophrenia and as a sleep aid for depression. It is or has been marketed under brand names including Dipiperon, Dipiperal, Piperonil, Piperonyl, and Propitan. Pipamperone was discovered at Janssen Pharmaceutica in 1961, and entered clinical trials in the United States in 1963.
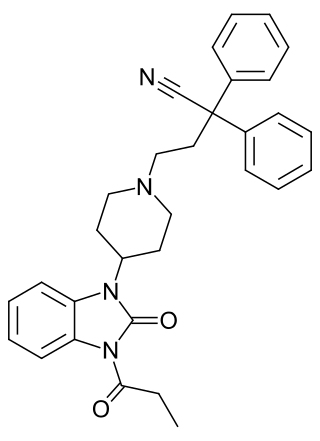
Bezitramide is an opioid analgesic. Bezitramide itself is a prodrug which is readily hydrolyzed in the gastrointestinal tract to its active metabolite, despropionyl-bezitramide. Bezitramide was discovered at Janssen Pharmaceutica in 1961. It is most commonly marketed under the trade name Burgodin.
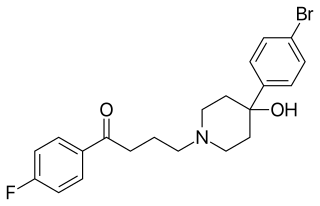
Bromperidol, sold under the brand names Bromidol and Impromen among others, is a typical antipsychotic of the butyrophenone group which is used in the treatment of schizophrenia. It was discovered at Janssen Pharmaceutica in 1966. An ester prodrug, bromperidol decanoate, is a long-acting form of bromperidol used as a depot injectable.
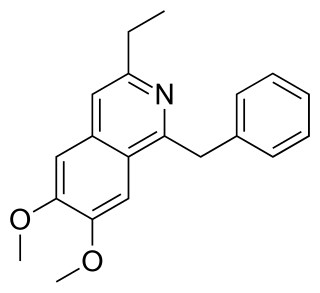
Moxaverine has been used in therapy based on the direct vasodilatory effect of the drug, a phosphodiesterase inhibitor, and on its influence on the rheological properties of red blood cells.

Metaclazepam is a drug which is a benzodiazepine derivative. It is a relatively selective anxiolytic with less sedative or muscle relaxant properties than other benzodiazepines such as diazepam or bromazepam. It has an active metabolite N-desmethylmetaclazepam, which is the main metabolite of metaclazepam. There is no significant difference in metabolism between younger and older individuals.

Propiram is a partial mu opioid receptor agonist and weak mu antagonist analgesic from the ampromide family of drugs related to other drugs such as phenampromide and diampromide. It was invented in 1963 in the United Kingdom by Bayer but was not widely marketed, although it saw some limited clinical use, especially in dentistry. Propiram reached Phase III clinical trials in the United States and Canada.

Mabuterol is a selective β2 adrenoreceptor agonist.

Zatosetron (LY-277,359) is a drug which acts as an antagonist at the 5HT3 receptor It is orally active and has a long duration of action, producing antinauseant effects but without stimulating the rate of gastrointestinal transport. It is also an effective anxiolytic in both animal studies and human trials, although with some side effects at higher doses.

Lerisetron (code name F-0930-RS) is a drug which acts as an antagonist at the 5-HT3 receptor. It is a potent antiemetic and was in clinical trials for the treatment of nausea associated with cancer chemotherapy.

Clopenthixol (Sordinol), also known as clopentixol, is a typical antipsychotic drug of the thioxanthene class. It was introduced by Lundbeck in 1961.

Pipotiazine (Piportil), also known as pipothiazine, is a typical antipsychotic of the phenothiazine class used in the United Kingdom and other countries for the treatment of schizophrenia. Its properties are similar to those of chlorpromazine. A 2004 systematic review investigated its efficacy for people with schizophrenia:

Fluperlapine, also known as fluoroperlapine, is a morphanthridine (11H-dibenzo[b,e]azepine) atypical antipsychotic with additional antidepressant and sedative effects. It was first synthesized in 1979, and then subsequently studied in animals and humans in 1984 and beyond, but despite demonstrating efficacy in the treatment of a variety of medical conditions including schizophrenia, psychosis associated with Parkinson's disease, depressive symptoms, and dystonia, it was never marketed. This was perhaps due to its capacity for producing potentially life-threatening agranulocytosis, similarly to clozapine, which it closely resembles both structurally and pharmacologically.
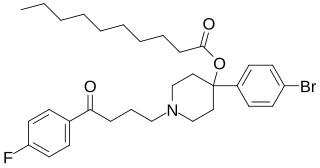
Bromperidol decanoate, sold under the brand names Bromidol Depot, Bromodol Decanoato, and Impromen Decanoas, is an antipsychotic which has been marketed in Europe and Latin America. It is an antipsychotic ester and long-acting prodrug of bromperidol which is administered by depot intramuscular injection once every 4 weeks.

Oxyprothepin decanoate, sold under the brand name Meclopin, is a typical antipsychotic which was used in the treatment of schizophrenia in the Czech Republic but is no longer marketed. It is administered by depot injection into muscle. The medication has an approximate duration of 2 to 3 weeks. The history of oxyprothepin decanoate has been reviewed.
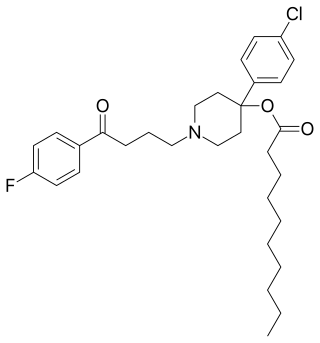
An antipsychotic ester is an ester of an antipsychotic. They are used clinically as prodrugs to increase fat solubility and thereby prolong duration when antipsychotics are used as depot injectables.



















