This article includes a list of general references, but it lacks sufficient corresponding inline citations .(November 2022) |
Amino esters are a class of local anesthetics. They are named for their ester bond and are unlike amide local anaesthetics.
This article includes a list of general references, but it lacks sufficient corresponding inline citations .(November 2022) |
Amino esters are a class of local anesthetics. They are named for their ester bond and are unlike amide local anaesthetics.
Structurally, amino esters consist of three molecular components:
The chemical linkage between the lipophilic part and the intermediate chain can be of the amide-type or the ester-type, and is the general basis for the current classification of local anesthetics.
Amino esters, in reference to anesthetic agents, are rapidly metabolized in the plasma by butyrylcholinesterase to para-aminobenzoic acid derivatives, then excreted in the urine. This suggests their very short half lives. Allergy is more likely to occur with ester-type agents, as opposed to amide-type.
Amino ester-type include:

In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is R−COOH or R−CO2H, with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 of one alpha-amino acid and N2 of another, along a peptide or protein chain.

Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthesis is most commonly performed by ribosomes in cells. Peptides can also be synthesized in the laboratory. Protein primary structures can be directly sequenced, or inferred from DNA sequences.

A local anesthetic (LA) is a medication that causes absence of pain sensation. In the context of surgery, a local anesthetic creates an absence of pain in a specific location of the body without a loss of consciousness, as opposed to a general anesthetic. When it is used on specific nerve pathways, paralysis also can be achieved.
4-Aminobenzoic acid (also known as para-aminobenzoic acid or PABA because the two functional groups are attached to the benzene ring across from one another in the para position) is an organic compound with the formula H2NC6H4CO2H. PABA is a white solid, although commercial samples can appear gray. It is slightly soluble in water. It consists of a benzene ring substituted with amino and carboxyl groups. The compound occurs extensively in the natural world.

An anesthetic or anaesthetic is a drug used to induce anesthesia — in other words, to result in a temporary loss of sensation or awareness. They may be divided into two broad classes: general anesthetics, which result in a reversible loss of consciousness, and local anesthetics, which cause a reversible loss of sensation for a limited region of the body without necessarily affecting consciousness.

In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl group of one amino acid to the amino group of another. Protecting group strategies are usually necessary to prevent undesirable side reactions with the various amino acid side chains. Chemical peptide synthesis most commonly starts at the carboxyl end of the peptide (C-terminus), and proceeds toward the amino-terminus (N-terminus). Protein biosynthesis in living organisms occurs in the opposite direction.

Bupivacaine, marketed under the brand name Marcaine among others, is a medication used to decrease feeling in a specific area. In nerve blocks, it is injected around a nerve that supplies the area, or into the spinal canal's epidural space. It is available mixed with a small amount of epinephrine to increase the duration of its action. It typically begins working within 15 minutes and lasts for 2 to 8 hours.
The peptidyl transferase is an aminoacyltransferase as well as the primary enzymatic function of the ribosome, which forms peptide bonds between adjacent amino acids using tRNAs during the translation process of protein biosynthesis. The substrates for the peptidyl transferase reaction are two tRNA molecules, one bearing the growing peptide chain and the other bearing the amino acid that will be added to the chain. The peptidyl chain and the amino acids are attached to their respective tRNAs via ester bonds to the O atom at the CCA-3' ends of these tRNAs. Peptidyl transferase is an enzyme that catalyzes the addition of an amino acid residue in order to grow the polypeptide chain in protein synthesis. It is located in the large ribosomal subunit, where it catalyzes the peptide bond formation. It is composed entirely of RNA. The alignment between the CCA ends of the ribosome-bound peptidyl tRNA and aminoacyl tRNA in the peptidyl transferase center contribute to its ability to catalyze these reactions. This reaction occurs via nucleophilic displacement. The amino group of the aminoacyl tRNA attacks the terminal carboxyl group of the peptidyl tRNA. Peptidyl transferase activity is carried out by the ribosome. Peptidyl transferase activity is not mediated by any ribosomal proteins but by ribosomal RNA (rRNA), a ribozyme. Ribozymes are the only enzymes which are not made up of proteins, but ribonucleotides. All other enzymes are made up of proteins. This RNA relic is the most significant piece of evidence supporting the RNA World hypothesis.
Nucleophilic acyl substitution describes a class of substitution reactions involving nucleophiles and acyl compounds. In this type of reaction, a nucleophile – such as an alcohol, amine, or enolate – displaces the leaving group of an acyl derivative – such as an acid halide, anhydride, or ester. The resulting product is a carbonyl-containing compound in which the nucleophile has taken the place of the leaving group present in the original acyl derivative. Because acyl derivatives react with a wide variety of nucleophiles, and because the product can depend on the particular type of acyl derivative and nucleophile involved, nucleophilic acyl substitution reactions can be used to synthesize a variety of different products.

The Weinreb–Nahm ketone synthesis is a chemical reaction used in organic chemistry to make carbon–carbon bonds. It was discovered in 1981 by Steven M. Weinreb and Steven Nahm as a method to synthesize ketones. The original reaction involved two subsequent nucleophilic acyl substitutions: the conversion of an acid chloride with N,O-Dimethylhydroxylamine, to form a Weinreb–Nahm amide, and subsequent treatment of this species with an organometallic reagent such as a Grignard reagent or organolithium reagent. Nahm and Weinreb also reported the synthesis of aldehydes by reduction of the amide with an excess of lithium aluminum hydride.
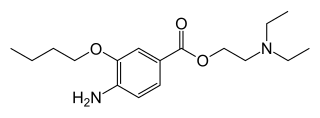
Oxybuprocaine (INN), also known as benoxinate or BNX, is an ester-type local anesthetic, which is used especially in ophthalmology and otolaryngology. Oxybuprocaine is sold by Novartis under the brand names Novesine or Novesin.
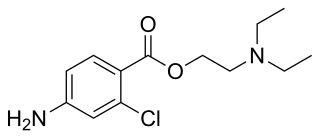
Chloroprocaine is a local anesthetic given by injection during surgical procedures and labor and delivery. Chloroprocaine vasodilates; this is in contrast to cocaine which vasoconstricts. Chloroprocaine is an ester anesthetic.

Levobupivacaine (rINN) is a local anaesthetic drug indicated for minor and major surgical anaesthesia and pain management. It is a long-acting amide-type local anaesthetic that blocks nerve impulses by inhibiting sodium ion influx into the nerve cells. Levobupivacaine is the S-enantiomer of racemic bupivacaine and therefore similar in pharmacological effects. The drug typically starts taking effect within 15 minutes and can last up to 16 hours depending on factors such as site of administration and dosage.
Gusperimus is an immunosuppressive drug. It is a derivative of the naturally occurring HSP70 inhibitor spergualin, and inhibits the interleukin-2-stimulated maturation of T cells to the S and G2/M phases and the polarization of the T cells into IFN-gamma-secreting Th1 effector T cells, resulting in the inhibition of growth of activated naive CD4 T cells.
Carbamic acid, which might also be called aminoformic acid or aminocarboxylic acid, is the chemical compound with the formula H2NCOOH. It can be obtained by the reaction of ammonia NH3 and carbon dioxide CO2 at very low temperatures, which also yields ammonium carbamate [NH4]+[NH2CO2]−. The compound is stable only up to about 250 K (−23 °C); at higher temperatures it decomposes into those two gases. The solid apparently consists of dimers, with the two molecules connected by hydrogen bonds between the two carboxyl groups –COOH.
Dental anesthesia is the application of anesthesia to dentistry. It includes local anesthetics, sedation, and general anesthesia.

Butacaine is a white crystalline ester used as a local anesthetic.
Dibucaine, also known as cinchocaine, is an amino amide local anesthetic. When administered to humans intravenously, it is capable of inhibiting the plasma cholinesterase (butyrylcholinesterase) enzyme. The dibucaine number is used to differentiate individuals who have substitution mutations of the enzyme's gene, resulting in decreased enzyme function.