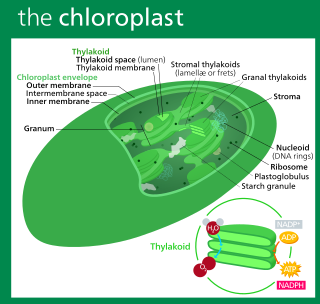
A chloroplast is a type of organelle known as a plastid that conducts photosynthesis mostly in plant and algal cells. Chloroplasts have a high concentration of chlorophyll pigments which capture the energy from sunlight and convert it to chemical energy and release oxygen. The chemical energy created is then used to make sugar and other organic molecules from carbon dioxide in a process called the Calvin cycle. Chloroplasts carry out a number of other functions, including fatty acid synthesis, amino acid synthesis, and the immune response in plants. The number of chloroplasts per cell varies from one, in some unicellular algae, up to 100 in plants like Arabidopsis and wheat.

Plant cells are the cells present in green plants, photosynthetic eukaryotes of the kingdom Plantae. Their distinctive features include primary cell walls containing cellulose, hemicelluloses and pectin, the presence of plastids with the capability to perform photosynthesis and store starch, a large vacuole that regulates turgor pressure, the absence of flagella or centrioles, except in the gametes, and a unique method of cell division involving the formation of a cell plate or phragmoplast that separates the new daughter cells.

Starch or amylum is a polymeric carbohydrate consisting of numerous glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants for energy storage. Worldwide, it is the most common carbohydrate in human diets, and is contained in large amounts in staple foods such as wheat, potatoes, maize (corn), rice, and cassava (manioc).

In vascular plants, the roots are the organs of a plant that are modified to provide anchorage for the plant and take in water and nutrients into the plant body, which allows plants to grow taller and faster. They are most often below the surface of the soil, but roots can also be aerial or aerating, that is, growing up above the ground or especially above water.
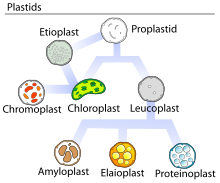
A plastid is a membrane-bound organelle found in the cells of plants, algae, and some other eukaryotic organisms. Plastids are considered to be intracellular endosymbiotic cyanobacteria.
Leucoplasts are a category of plastid and as such are organelles found in plant cells. They are non-pigmented, in contrast to other plastids such as the chloroplast.

Chromoplasts are plastids, heterogeneous organelles responsible for pigment synthesis and storage in specific photosynthetic eukaryotes. It is thought that like all other plastids including chloroplasts and leucoplasts they are descended from symbiotic prokaryotes.

Elaioplasts are one of the three possible forms of leucoplasts, sometimes broadly referred to as such. The main function of elaioplasts is synthesis and storage of fatty acids, terpenes, and other lipids, and they can be found in the embryonic leaves of certain plants, as well as the anthers of many flowering plants.

The endodermis is the innermost layer of cortex in land plants. It is a cylinder of compact living cells, the radial walls of which are impregnated with hydrophobic substances to restrict apoplastic flow of water to the inside. The endodermis is the boundary between the cortex and the stele.

Phytochromes are a class of photoreceptor proteins found in plants, bacteria and fungi. They respond to light in the red and far-red regions of the visible spectrum and can be classed as either Type I, which are activated by far-red light, or Type II that are activated by red light. Recent advances have suggested that phytochromes also act as temperature sensors, as warmer temperatures enhance their de-activation. All of these factors contribute to the plant's ability to germinate.

Jasmonate (JA) and its derivatives are lipid-based plant hormones that regulate a wide range of processes in plants, ranging from growth and photosynthesis to reproductive development. In particular, JAs are critical for plant defense against herbivory and plant responses to poor environmental conditions and other kinds of abiotic and biotic challenges. Some JAs can also be released as volatile organic compounds (VOCs) to permit communication between plants in anticipation of mutual dangers.

Abscisic acid is a plant hormone. ABA functions in many plant developmental processes, including seed and bud dormancy, the control of organ size and stomatal closure. It is especially important for plants in the response to environmental stresses, including drought, soil salinity, cold tolerance, freezing tolerance, heat stress and heavy metal ion tolerance.

Coleoptile is the pointed protective sheath covering the emerging shoot in monocotyledons such as grasses in which few leaf primordia and shoot apex of monocot embryo remain enclosed. The coleoptile protects the first leaf as well as the growing stem in seedlings and eventually, allows the first leaf to emerge. Coleoptiles have two vascular bundles, one on either side. Unlike the flag leaves rolled up within, the pre-emergent coleoptile does not accumulate significant protochlorophyll or carotenoids, and so it is generally very pale. Some preemergent coleoptiles do, however, accumulate purple anthocyanin pigments.

Hydrotropism is a plant's growth response in which the direction of growth is determined by a stimulus or gradient in water concentration. A common example is a plant root growing in humid air bending toward a higher relative humidity level.

Gravitropism is a coordinated process of differential growth by a plant in response to gravity pulling on it. It also occurs in fungi. Gravity can be either "artificial gravity" or natural gravity. It is a general feature of all higher and many lower plants as well as other organisms. Charles Darwin was one of the first to scientifically document that roots show positive gravitropism and stems show negative gravitropism. That is, roots grow in the direction of gravitational pull and stems grow in the opposite direction. This behavior can be easily demonstrated with any potted plant. When laid onto its side, the growing parts of the stem begin to display negative gravitropism, growing upwards. Herbaceous (non-woody) stems are capable of a degree of actual bending, but most of the redirected movement occurs as a consequence of root or stem growth outside. The mechanism is based on the Cholodny–Went model which was proposed in 1927, and has since been modified. Although the model has been criticized and continues to be refined, it has largely stood the test of time.

Proteinoplasts are specialized organelles found only in plant cells. Proteinoplasts belong to a broad category of organelles known as plastids. Plastids are specialized double-membrane organelles found in plant cells. Plastids perform a variety of functions such as metabolism of energy, and biological reactions. There are multiple types of plastids recognized including Leucoplasts, Chromoplasts, and Chloroplasts. Plastids are broken up into different categories based on characteristics such as size, function and physical traits. Chromoplasts help to synthesize and store large amounts of carotenoids. Chloroplasts are photosynthesizing structures that help to make light energy for the plant. Leucoplasts are a colorless type of plastid which means that no photosynthesis occurs here. The colorless pigmentation of the leucoplast is due to not containing the structural components of thylakoids unlike what is found in chloroplasts and chromoplasts that gives them their pigmentation. From leucoplasts stems the subtype, proteinoplasts, which contain proteins for storage. They contain crystalline bodies of protein and can be the sites of enzyme activity involving those proteins. Proteinoplasts are found in many seeds, such as brazil nuts, peanuts and pulses. Although all plastids contain high concentrations of protein, proteinoplasts were identified in the 1960s and 1970s as having large protein inclusions that are visible with both light microscopes and electron microscopes. Other subtypes of Leucoplasts include amyloplast, and elaioplasts. Amyloplasts help to store and synthesize starch molecules found in plants, while elaioplasts synthesize and store lipids in plant cells.

Statocytes are gravity-sensing (gravitropic) cells in higher plants. They contain amyloplasts-statoliths – starch-filled amyloplastic organelles – which sediment at the lowest part of the cells. In the roots, sedimentation of the statoliths towards the lower part of the statocytes constitutes a signal for the production and redistribution of auxin. When stems or roots are not exactly aligned with the gravity vector, statoliths move and adjust to gravity. This is followed by a triggering of the asymmetrical distribution of auxin that causes the curvature and growth of stems against the gravity vector, as well as growth of roots along the gravity vector. Statocytes are present in the elongating region of coleoptiles, shoots and inflorescence stems. In roots, the root cap is the only place where sedimentation is observed, and only the central columella cells of the root cap serve as gravity-sensing statocytes. They can initiate differential growth patterns, bending the root towards the vertical axis.

In biology, phototropism is the growth of an organism in response to a light stimulus. Phototropism is most often observed in plants, but can also occur in other organisms such as fungi. The cells on the plant that are farthest from the light contain a hormone called auxin that reacts when phototropism occurs. This causes the plant to have elongated cells on the furthest side from the light. Phototropism is one of the many plant tropisms, or movements, which respond to external stimuli. Growth towards a light source is called positive phototropism, while growth away from light is called negative phototropism. Negative phototropism is not to be confused with skototropism, which is defined as the growth towards darkness, whereas negative phototropism can refer to either the growth away from a light source or towards the darkness. Most plant shoots exhibit positive phototropism, and rearrange their chloroplasts in the leaves to maximize photosynthetic energy and promote growth. Some vine shoot tips exhibit negative phototropism, which allows them to grow towards dark, solid objects and climb them. The combination of phototropism and gravitropism allow plants to grow in the correct direction.

Randy O. Wayne is an associate professor of plant biology at Cornell University. Along with his former colleague Peter K. Hepler, Wayne established the role of calcium in regulating plant growth. Their 1985 article Calcium and Plant Development was awarded the "Citation Classic" award from Current Contents magazine. They researched how plant cells sense gravity through pressure, the water permeability of plant membranes, light microscopy, as well as the effects of calcium on plant development. Wayne authored two textbooks, including Plant Cell Biology: From Astronomy to Zoology and Light and Video Microscopy.

Pal Maliga is a plant molecular biologist. He is Distinguished Professor of Plant Biology and Laboratory Director at the Waksman Institute of Microbiology, Rutgers University. He is known for developing the technology of chloroplast genome engineering in land plants and its applications in basic science and biotechnology.