Bromine is a chemical element; it has symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig and Antoine Jérôme Balard, its name was derived from Ancient Greek βρῶμος (bromos) 'stench', referring to its sharp and pungent smell.

The halogens are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would exclude tennessine as its chemistry is unknown and is theoretically expected to be more like that of gallium. In the modern IUPAC nomenclature, this group is known as group 17.

The haloalkanes are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula "RX" where R is an alkyl or substituted alkyl group and X is a halogen.

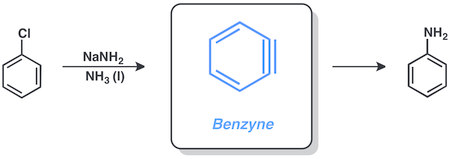
Aniline is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans.
In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens. Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.

An acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.
The Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts using copper salts as reagents or catalysts. It is an example of a radical-nucleophilic aromatic substitution. The Sandmeyer reaction provides a method through which one can perform unique transformations on benzene, such as halogenation, cyanation, trifluoromethylation, and hydroxylation.

A nucleophilic aromatic substitution (SNAr) is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring. Aromatic rings are usually nucleophilic, but some aromatic compounds do undergo nucleophilic substitution. Just as normally nucleophilic alkenes can be made to undergo conjugate substitution if they carry electron-withdrawing substituents, so normally nucleophilic aromatic rings also become electrophilic if they have the right substituents.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. The parent compound where R is hydrogen, is diazenylium.

Thiophenol is an organosulfur compound with the formula C6H5SH, sometimes abbreviated as PhSH. This foul-smelling colorless liquid is the simplest aromatic thiol. The chemical structures of thiophenol and its derivatives are analogous to phenols, where the oxygen atom in the hydroxyl group (-OH) bonded to the aromatic ring in phenol is replaced by a sulfur atom. The prefix thio- implies a sulfur-containing compound and when used before a root word name for a compound which would normally contain an oxygen atom, in the case of 'thiol' that the alcohol oxygen atom is replaced by a sulfur atom.

The Finkelstein reaction, named after the German chemist Hans Finkelstein, is a type of SN2 reaction that involves the exchange of one halogen atom for another. It is an equilibrium reaction, but the reaction can be driven to completion by exploiting the differential solubility of various halide salts, or by using a large excess of the desired halide.
Bromobenzenes are a group of aryl bromides/halobenzenes consisting of one or more bromine atoms as substituents on a benzene core. They have the formula C6H6–nBrn, where n = 1–6 is the number of bromine atoms. Depending on the number of bromine substituents, there may be several constitutional isomers possible.
Bromine compounds are compounds containing the element bromine (Br). These compounds usually form the -1, +1, +3 and +5 oxidation states. Bromine is intermediate in reactivity between chlorine and iodine, and is one of the most reactive elements. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. This can be seen from the standard electrode potentials of the X2/X− couples (F, +2.866 V; Cl, +1.395 V; Br, +1.087 V; I, +0.615 V; At, approximately +0.3 V). Bromination often leads to higher oxidation states than iodination but lower or equal oxidation states to chlorination. Bromine tends to react with compounds including M–M, M–H, or M–C bonds to form M–Br bonds.
Iodine compounds are compounds containing the element iodine. Iodine can form compounds using multiple oxidation states. Iodine is quite reactive, but it is much less reactive than the other halogens. For example, while chlorine gas will halogenate carbon monoxide, nitric oxide, and sulfur dioxide, iodine will not do so. Furthermore, iodination of metals tends to result in lower oxidation states than chlorination or bromination; for example, rhenium metal reacts with chlorine to form rhenium hexachloride, but with bromine it forms only rhenium pentabromide and iodine can achieve only rhenium tetraiodide. By the same token, however, since iodine has the lowest ionisation energy among the halogens and is the most easily oxidised of them, it has a more significant cationic chemistry and its higher oxidation states are rather more stable than those of bromine and chlorine, for example in iodine heptafluoride.
In chemistry, a sulfonyl halide consists of a sulfonyl group singly bonded to a halogen atom. They have the general formula RSO2X, where X is a halogen. The stability of sulfonyl halides decreases in the order fluorides > chlorides > bromides > iodides, all four types being well known. The sulfonyl chlorides and fluorides are of dominant importance in this series.
The Balz–Schiemann reaction is a chemical reaction in which a primary aromatic amine is transformed to an aryl fluoride via a diazonium tetrafluoroborate intermediate. This reaction is a traditional route to fluorobenzene and some related derivatives, including 4-fluorobenzoic acid.
A cyanogen halide is a molecule consisting of cyanide and a halogen. Cyanogen halides are chemically classified as pseudohalogens.
Organobromine chemistry is the study of the synthesis and properties of organobromine compounds, also called organobromides, which are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane.
Electrophilic aromatic substitution (SEAr) is an organic reaction in which an atom that is attached to an aromatic system is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic nitration, aromatic halogenation, aromatic sulfonation, alkylation Friedel–Crafts reaction and acylation Friedel–Crafts reaction.
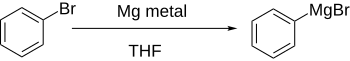
In organometallic chemistry, metal–halogen exchange is a fundamental reaction that converts an organic halide into an organometallic product. The reaction commonly involves the use of electropositive metals and organochlorides, bromides, and iodides. Particularly well-developed is the use of metal–halogen exchange for the preparation of organolithium compounds.