In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries a partial positive charge, or have an atom that does not have an octet of electrons.
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reactions, it resembles benzene. Compounds analogous to thiophene include furan (C4H4O), selenophene (C4H4Se) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring.
In organic chemistry, an acyl chloride is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.
In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens. Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.
In organic chemistry, an aryl halide is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. Haloarenes are different from haloalkanes because they exhibit many differences in methods of preparation and properties. The most important members are the aryl chlorides, but the class of compounds is so broad that there are many derivatives and applications.

In organic chemistry, an acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.

In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols and nitric acid. The difference between the resulting molecular structures of nitro compounds and nitrates is that the nitrogen atom in nitro compounds is directly bonded to a non-oxygen atom, whereas in nitrate esters, the nitrogen is bonded to an oxygen atom that in turn usually is bonded to a carbon atom.

Thionyl chloride is an inorganic compound with the chemical formula SOCl2. It is a moderately volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately 45,000 tonnes per year being produced during the early 1990s, but is occasionally also used as a solvent. It is toxic, reacts with water, and is also listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons.

Chlorobenzene (abbreviated PhCl) is an aryl chloride and the simplest of the chlorobenzenes, consisting of a benzene ring substituted with one chlorine atom. Its chemical formula is C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals.

Sulfur dichloride is the chemical compound with the formula SCl2. This cherry-red liquid is the simplest sulfur chloride and one of the most common, and it is used as a precursor to organosulfur compounds. It is a highly corrosive and toxic substance, and it reacts on contact with water to form chlorine-containing acids.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most common oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.
Magnesium compounds are compounds formed by the element magnesium (Mg). These compounds are important to industry and biology, including magnesium carbonate, magnesium chloride, magnesium citrate, magnesium hydroxide, magnesium oxide, magnesium sulfate, and magnesium sulfate heptahydrate.
In inorganic chemistry, sulfonyl halide groups occur when a sulfonyl functional group is singly bonded to a halogen atom. They have the general formula RSO2X, where X is a halogen. The stability of sulfonyl halides decreases in the order fluorides > chlorides > bromides > iodides, all four types being well known. The sulfonyl chlorides and fluorides are of dominant importance in this series.

Thiophosphoryl chloride is an inorganic compound with the chemical formula PSCl3. It is a colorless pungent smelling liquid that fumes in air. It is synthesized from phosphorus chloride and used to thiophosphorylate organic compounds, such as to produce insecticides.
Methanesulfonyl chloride is an organosulfur compound with the formula CH3SO2Cl. Using the organic pseudoelement symbol Ms for the methanesulfonyl group CH3SO2–, it is frequently abbreviated MsCl in reaction schemes or equations. It is a colourless liquid that dissolves in polar organic solvents but is reactive toward water, alcohols, and many amines. The simplest organic sulfonyl chloride, it is used to make methanesulfonates and to generate the elusive molecule sulfene.
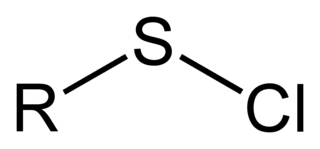
In organosulfur chemistry, a sulfenyl chloride is a functional group with the connectivity R−S−Cl, where R is alkyl or aryl. Sulfenyl chlorides are reactive compounds that behave as sources of RS+. They are used in the formation of RS−N and RS−O bonds. According to IUPAC nomenclature they are named as alkyl thiohypochlorites, i.e. esters of thiohypochlorous acid.

4-Nitrochlorobenzene is the organic compound with the formula ClC6H4NO2. It is a pale yellow solid. 4-Nitrochlorobenzene is a common intermediate in the production of a number of industrially useful compounds, including antioxidants commonly found in rubber. Other isomers with the formula ClC6H4NO2 include 2-nitrochlorobenzene and 3-nitrochlorobenzene.
Dimethyl disulfide (DMDS) is an organic chemical compound with the molecular formula CH3SSCH3. It is a flammable liquid with an unpleasant, garlic-like odor. The compound is colorless although impure samples often appear yellowish.
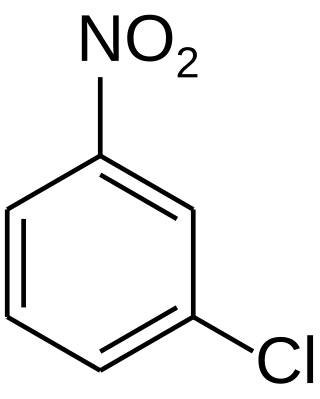
3-Nitrochlorobenzene is an organic compound with the formula C6H4ClNO2. It is a yellow crystalline solid that is important as a precursor to other compounds due to the two reactive sites present on the molecule.

Hexafluorothioacetone is an organic perfluoro thione compound with formula CF3CSCF3. At standard conditions it is a blue gas.














