
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula R−C(=O)−NR′R″, where R, R', and R″ represent any group, typically organyl groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid with the hydroxyl group replaced by an amine group ; or, equivalently, an acyl (alkanoyl) group joined to an amine group.
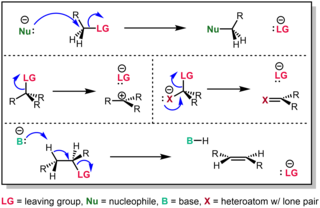
In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited to a fragment that departs with a pair of electrons in heterolytic bond cleavage. In this usage, a leaving group is a less formal but more commonly used synonym of the term nucleofuge. In this context, leaving groups are generally anions or neutral species, departing from neutral or cationic substrates, respectively, though in rare cases, cations leaving from a dicationic substrate are also known.
In organic chemistry, a carbanion is an anion in which carbon is negatively charged.

In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant to hydrolysis. In terms of commercial applications, imides are best known as components of high-strength polymers, called polyimides. Inorganic imides are also known as solid state or gaseous compounds, and the imido group (=NH) can also act as a ligand.
In chemistry, a superacid (according to the original definition) is an acid with an acidity greater than that of 100% pure sulfuric acid (H2SO4), which has a Hammett acidity function (H0) of −12. According to the modern definition, a superacid is a medium in which the chemical potential of the proton is higher than in pure sulfuric acid. Commercially available superacids include trifluoromethanesulfonic acid (CF3SO3H), also known as triflic acid, and fluorosulfuric acid (HSO3F), both of which are about a thousand times stronger (i.e. have more negative H0 values) than sulfuric acid. Most strong superacids are prepared by the combination of a strong Lewis acid and a strong Brønsted acid. A strong superacid of this kind is fluoroantimonic acid. Another group of superacids, the carborane acid group, contains some of the strongest known acids. Finally, when treated with anhydrous acid, zeolites (microporous aluminosilicate minerals) will contain superacidic sites within their pores. These materials are used on massive scale by the petrochemical industry in the upgrading of hydrocarbons to make fuels.
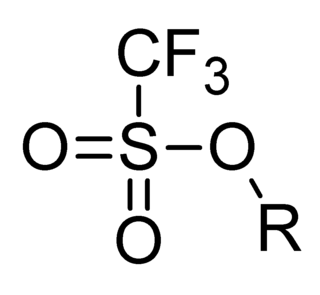
In organic chemistry, triflate, is a functional group with the formula R−OSO2CF3 and structure R−O−S(=O)2−CF3. The triflate group is often represented by −OTf, as opposed to −Tf, which is the triflyl group, R−SO2CF3. For example, n-butyl triflate can be written as CH3CH2CH2CH2OTf.
The Hofmann rearrangement is the organic reaction of a primary amide to a primary amine with one less carbon atom. The reaction involves oxidation of the nitrogen followed by rearrangement of the carbonyl and nitrogen to give an isocyanate intermediate. The reaction can form a wide range of products, including alkyl and aryl amines.

Fluorosulfuric acid (IUPAC name: sulfurofluoridic acid) is the inorganic compound with the chemical formula HSO3F. It is one of the strongest acids commercially available. It is a tetrahedral molecule and is closely related to sulfuric acid, H2SO4, substituting a fluorine atom for one of the hydroxyl groups. It is a colourless liquid, although commercial samples are often yellow.

Fluoroantimonic acid is a mixture of hydrogen fluoride and antimony pentafluoride, containing various cations and anions. This mixture is a superacid that, in terms of corrosiveness, is trillions of times stronger than 100% sulfuric acid in terms of its protonating ability measured by Hammett function. It even protonates some hydrocarbons to afford pentacoordinate carbocations. Fluoroantimonic acid is corrosive. Like its precursor hydrogen fluoride, it attacks glass, but can be stored in containers lined with PTFE (Teflon) or PFA.
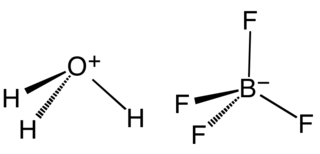
Fluoroboric acid or tetrafluoroboric acid is an inorganic compound with the simplified chemical formula H+[BF4]−. Unlike other strong acids like H2SO4 or HClO4, the pure tetrafluoroboric acid does not exist. The term "fluoroboric acid" refers to a range of chemical compounds, depending on the solvent. The H+ in the simplified formula of fluoroboric acid represents the solvated proton. The solvent can be any suitable Lewis base. For instance, if the solvent is water, fluoroboric acid can be represented by the formula [H3O]+[BF4]−, although more realistically, several water molecules solvate the proton: [H(H2O)n]+[BF4]−. The ethyl ether solvate is also commercially available, where the fluoroboric acid can be represented by the formula [H( 2O)n]+[BF4]−, where n is most likely 2.

Lithium bis(trimethylsilyl)amide is a lithiated organosilicon compound with the formula LiN(Si(CH3)3)2. It is commonly abbreviated as LiHMDS or Li(HMDS) (lithium hexamethyldisilazide - a reference to its conjugate acid HMDS) and is primarily used as a strong non-nucleophilic base and as a ligand. Like many lithium reagents, it has a tendency to aggregate and will form a cyclic trimer in the absence of coordinating species.

In organic chemistry, the triflyl group is a functional group with the formula R−SO2CF3 and structure R−S(=O)2−CF3. The triflyl group is often represented by –Tf.
A metal triflimidate M(NTf2)n in organic chemistry is a metal salt or complex of triflimidic acid and used as a catalyst.
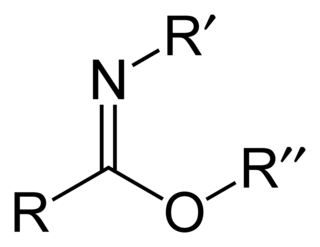
Carboximidates are organic compounds, which can be thought of as esters formed between a imidic acid and an alcohol, with the general formula R-C(=NR')OR".
Acid strength is the tendency of an acid, symbolised by the chemical formula , to dissociate into a proton, , and an anion, . The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions.

Carborane acidsH(CXB
11Y
5Z
6) (X, Y, Z = H, Alk, F, Cl, Br, CF3) are a class of superacids, some of which are estimated to be at least one million times stronger than 100% pure sulfuric acid in terms of their Hammett acidity function values (H0 ≤ –18) and possess computed pKa values well below –20, establishing them as some of the strongest known Brønsted acids. The best-studied example is the highly chlorinated derivative H(CHB
11Cl
11). The acidity of H(CHB
11Cl
11) was found to vastly exceed that of triflic acid, CF
3SO
3H, and bistriflimide, (CF
3SO
2)
2NH, compounds previously regarded as the strongest isolable acids.
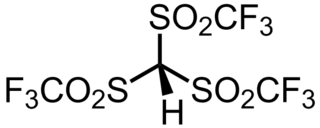
Triflidic acid (IUPAC name: tris[(trifluoromethyl)sulfonyl]methane, abbreviated formula: Tf3CH) is an organic superacid. It is one of the strongest known carbon acids and is among the strongest Brønsted acids in general, with an acidity exceeded only by the carborane acids. Notably, triflidic acid is estimated to have an acidity 104 times that of triflic acid (pKaaq ~ –14), as measured by its acid dissociation constant. It was first prepared in 1987 by Seppelt and Turowsky by the following route:
(1) Tf2CH2 + 2CH3MgBr → Tf2C(MgBr)2 + 2CH4
(2) Tf2C(MgBr)2 + TfF → Tf3C(MgBr) + MgBrF
(3) Tf3C(MgBr) + H2SO4 → Tf3CH + MgBrHSO4

Lithium bis(trifluoromethanesulfonyl)imide, often simply referred to as LiTFSI, is a hydrophilic salt with the chemical formula LiC2F6NO4S2. It is commonly used as Li-ion source in electrolytes for Li-ion batteries as a safer alternative to commonly used lithium hexafluorophosphate. It is made up of one Li cation and a bistriflimide anion.

Azanide is the IUPAC-sanctioned name for the anion NH−2. The term is obscure; derivatives of NH−2 are almost invariably referred to as amides, despite the fact that amide also refers to the organic functional group –C(=O)−NR2. The anion NH−2 is the conjugate base of ammonia, so it is formed by the self-ionization of ammonia. It is produced by deprotonation of ammonia, usually with strong bases or an alkali metal. Azanide has a H–N–H bond angle of 104.5°.
In chemistry, the term amide ( or or ) is a compound with the functional group RnE(=O)xNR2, where n and x may be 1 or 2, E is some element, and each R represents an organic group or hydrogen. It is a derivative of an oxoacid RnE(=O)xOH with an hydroxy group –OH replaced by an amine group –NR2.

















