
A lithium polymer battery, or more correctly lithium-ion polymer battery, is a rechargeable battery of lithium-ion technology using a polymer electrolyte instead of a liquid electrolyte. High conductivity semisolid (gel) polymers form this electrolyte. These batteries provide higher specific energy than other lithium battery types and are used in applications where weight is a critical feature, such as mobile devices, radio-controlled aircraft and some electric vehicles.

In chemistry, an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding. The compound is neutral overall, but consists of positively charged ions called cations and negatively charged ions called anions. These can be simple ions such as the sodium (Na+) and chloride (Cl−) in sodium chloride, or polyatomic species such as the ammonium (NH+
4) and carbonate (CO2−
3) ions in ammonium carbonate. Individual ions within an ionic compound usually have multiple nearest neighbours, so are not considered to be part of molecules, but instead part of a continuous three-dimensional network. Ionic compounds usually form crystalline structures when solid.

In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant to hydrolysis. In terms of commercial applications, imides are best known as components of high-strength polymers, called polyimides. Inorganic imides are also known as solid state or gaseous compounds, and the imido group (=NH) can also act as a ligand.

An ionic liquid (IL) is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below some arbitrary temperature, such as 100 °C (212 °F). While ordinary liquids such as water and gasoline are predominantly made of electrically neutral molecules, ionic liquids are largely made of ions. These substances are variously called liquid electrolytes, ionic melts, ionic fluids, fused salts, liquid salts, or ionic glasses.
Liquid–liquid extraction (LLE), also known as solvent extraction and partitioning, is a method to separate compounds or metal complexes, based on their relative solubilities in two different immiscible liquids, usually water (polar) and an organic solvent (non-polar). There is a net transfer of one or more species from one liquid into another liquid phase, generally from aqueous to organic. The transfer is driven by chemical potential, i.e. once the transfer is complete, the overall system of chemical components that make up the solutes and the solvents are in a more stable configuration. The solvent that is enriched in solute(s) is called extract. The feed solution that is depleted in solute(s) is called the raffinate. LLE is a basic technique in chemical laboratories, where it is performed using a variety of apparatus, from separatory funnels to countercurrent distribution equipment called as mixer settlers. This type of process is commonly performed after a chemical reaction as part of the work-up, often including an acidic work-up.
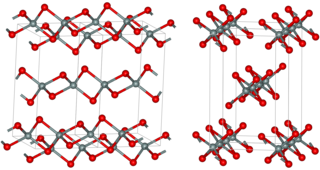
Silicon disulfide is the inorganic compound with the formula SiS2. Like silicon dioxide, this material is polymeric, but it adopts a 1-dimensional structure quite different from the usual forms of SiO2.
Bistriflimide, systematically known as bis(trifluoromethane)sulfonimide (or 'imidate', see below) and colloquially as TFSI or NTf2, is a non-coordinating anion with the chemical formula [(CF3SO2)2N]−. Its salts are typically referred to as being metal triflimidates.

Hexafluorophosphate is an anion with chemical formula of PF−
6. It is an octahedral species that imparts no color to its salts. PF−
6 is isoelectronic with sulfur hexafluoride, SF6, and the hexafluorosilicate dianion, SiF2−
6, and fluoroantimonate SbF−
6. Being poorly nucleophilic, hexafluorophosphate is classified as a non-coordinating anion.

Group 2 organometallic chemistry refers to the chemistry of compounds containing carbon bonded to any group 2 element. By far the most common group 2 organometallic compounds are the magnesium-containing Grignard reagents which are widely used in organic chemistry. Other organmetallic group 2 compounds are rare and are typically limited to academic interests.
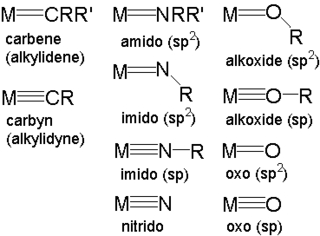
In Chemistry, a metal–ligand multiple bond describes the interaction of certain ligands with a metal with a bond order greater than one. Coordination complexes featuring multiply bonded ligands are of both scholarly and practical interest. Transition metal carbene complexes catalyze the olefin metathesis reaction. Metal oxo intermediates are pervasive in oxidation catalysis. oxygen evolving complex.

Tetrandrine, a bis-benzylisoquinoline alkaloid, is a calcium channel blocker. It has anti-inflammatory, immunologic and antiallergenic effects. It inhibits the degranulation of mast cells. It has a quinidine-like anti-arrhythmic effect. It is isolated from the plant Stephania tetrandra, and other Chinese and Japanese herbs. It has vasodilatory properties and can therefore reduce blood pressure. Tetrandrine may have potential use for the treatment of liver disease and liver cancer. Tetrandrine has potential therapeutic value to prevent excess scarring/fibrosis in conjunctiva following trabeculectomy or in patients with severe conjunctival inflammation. Tetrandrine has anti-inflammatory and anti-fibrogenic actions, which make tetrandrine and related compounds potentially useful in the treatment of lung silicosis, liver cirrhosis, and rheumatoid arthritis. Tetrandrine has also been shown to inhibit entry of Ebola virus into host cells in vitro and showed therapeutic efficacy against Ebola in preliminary studies on mice.
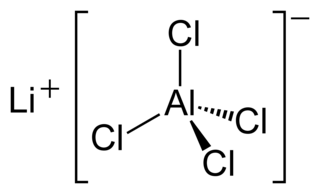
Lithium tetrachloroaluminate (LAC, lithium aluminium chloride) is an inorganic compound, a tetrachloroaluminate of lithium, with the formula LiAlCl4.
Nitrogen pentafluoride (NF5) is a theoretical compound of nitrogen and fluorine that is hypothesized to exist based on the existence of the pentafluorides of the atoms below nitrogen in the periodic table, such as phosphorus pentafluoride. Theoretical models of the nitrogen pentafluoride molecule are either a trigonal bipyramidal covalently bound molecule with symmetry group D3h, or NF+
4F−, which would be an ionic solid.

Perfluorobutanesulfonyl fluoride (nonafluorobutanesulfonyl fluoride, NfF) is a colorless, volatile liquid that is immiscible with water but soluble in common organic solvents. It is prepared by the electrochemical fluorination of sulfolane. NfF serves as an entry point to nonafluorobutanesulfonates (nonaflates), which are valuable as electrophiles in palladium catalyzed cross coupling reactions. As a perfluoroalkylsulfonylating agent, NfF offers the advantages of lower cost and greater stability over the more frequently used triflic anhydride. The fluoride leaving group is readily substituted by nucleophiles such as amines, phenoxides, and enolates, giving sulfonamides, aryl nonaflates, and alkenyl nonaflates, respectively. However, it is not attacked by water (in which it is stable at pH<12). Hydrolysis by barium hydroxide gives Ba(ONf)2, which upon treatment with sulfuric acid gives perfluorobutanesulfonic acid and insoluble barium sulfate.

Bromine azide is an explosive inorganic compound with the formula BrN3. It has been described as a crystal or a red liquid at room temperature. It is extremely sensitive to small variations in temperature and pressure, with explosions occurring at Δp ≥ 0.05 Torr and also upon crystallization, thus extreme caution must be observed when working with this reagent.
Ioliomics is a research discipline dealing with the studies of ions in liquids and stipulated with fundamental differences of ionic interactions. The name is a combination of IOns, LIquids and -OMICS. Ioliomics covers a broad research area concerning structure, properties and applications of ions involved in various biological and chemical systems. The concept of this research discipline is related to other comprehensive research fields, such as genomics, proteomics, glycomics, petroleomics, etc., where the suffix -omics is used for describing the comprehensiveness of data.

Lithium bis(trifluoromethanesulfonyl)imide, often simply referred to as LiTFSI, is a hydrophilic salt with the chemical formula LiC2F6NO4S2. It is commonly used as Li-ion source in electrolytes for Li-ion batteries as a safer alternative to commonly used lithium hexafluorophosphate. It is made up of one Li cation and a bistriflimide anion.

2,6-Diisopropylaniline is an organic compound with the formula H2NC6H3(CHMe2)2 (Me = CH3). It is a colorless liquid although, like many anilines, samples can appear yellow or brown. 2,6-Diisopropylaniline is a bulky aromatic amine that is often used to make ligands in coordination chemistry. The Schrock carbenes often are transition metal imido complexes derived from this aniline. Condensation with diacetylpyridine and acetylacetone gives, respectively, diiminopyridine and NacNac ligands.
The inorganic imides are compounds containing an ion composed of nitrogen bonded to hydrogen with formula HN2−. Organic imides have the NH group, and two single or one double covalent bond to other atoms. The imides are related to the inorganic amides (H2N−), the nitrides (N3−) and the nitridohydrides (N3−•H−).
The borate oxalates are chemical compounds containing borate and oxalate anions. Where the oxalate group is bound to the borate via oxygen, a more condensed anion is formed that balances less cations. These can be termed boro-oxalates, bis(oxalato)borates, or oxalatoborates or oxalate borates. The oxalatoborates are heterocyclic compounds with a ring containing -O-B-O-. Bis(oxalato)borates are spiro compounds with rings joined at the boron atom.















