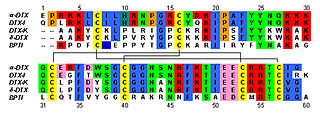
Dendrotoxins are a class of presynaptic neurotoxins produced by mamba snakes (Dendroaspis) that block particular subtypes of voltage-gated potassium channels in neurons, thereby enhancing the release of acetylcholine at neuromuscular junctions. Because of their high potency and selectivity for potassium channels, dendrotoxins have proven to be extremely useful as pharmacological tools for studying the structure and function of these ion channel proteins.
The shaker (Sh) gene, when mutated, causes a variety of atypical behaviors in the fruit fly, Drosophila melanogaster. Under ether anesthesia, the fly’s legs will shake ; even when the fly is unanaesthetized, it will exhibit aberrant movements. Sh-mutant flies have a shorter lifespan than regular flies; in their larvae, the repetitive firing of action potentials as well as prolonged exposure to neurotransmitters at neuromuscular junctions occurs.

Voltage-gated potassium channels (VGKCs) are transmembrane channels specific for potassium and sensitive to voltage changes in the cell's membrane potential. During action potentials, they play a crucial role in returning the depolarized cell to a resting state.

Maurotoxin is a peptide toxin from the venom of the Tunisian chactoid scorpion Scorpio maurus palmatus, from which it was first isolated and from which the chemical gets its name. It acts by blocking several types of voltage-gated potassium channel.

Kappa- Hefutoxin 1 and 2 are toxins from the venom of the Asian forest black scorpion Heterometrus fulvipes with a unique structure. It blocks the potassium channels Kv1.2 and Kv1.3 and slows the activation of Kv1.3.

Heteropodatoxins are peptide toxins from the venom of the giant crab spider Heteropoda venatoria, which block Kv4.2 voltage-gated potassium channels.

Potassium voltage-gated channel, shaker-related subfamily, member 5, also known as KCNA5 or Kv1.5, is a protein that in humans is encoded by the KCNA5 gene.

Potassium voltage-gated channel, shaker-related subfamily, member 3, also known as KCNA3 or Kv1.3, is a protein that in humans is encoded by the KCNA3 gene.
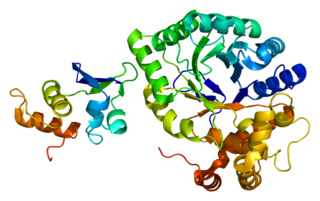
Voltage-gated potassium channel subunit beta-2 is a protein that in humans is encoded by the KCNAB2 gene.

Potassium voltage-gated channel subfamily E member 4, originally named MinK-related peptide 3 or MiRP3 when it was discovered, is a protein that in humans is encoded by the KCNE4 gene.

Cobatoxin is a toxin present in the venom of the scorpion Centruroides noxius. It blocks two potassium channel subtypes; voltage-gated and calcium-activated channels.

Heteroscodratoxin-1 is a neurotoxin produced by the venom glands of Heteroscodra maculata that shifts the activation threshold of voltage-gated potassium channels and the inactivation of Nav1.1 sodium channels to more positive potentials.

Pi3 toxin is a purified peptide derivative of the Pandinus imperator scorpion venom. It is a potent blocker of voltage-gated potassium channel, Kv1.3 and is closely related to another peptide found in the venom, Pi2.
BgK is a neurotoxin found within secretions of the sea anemone Bunodosomagranulifera which blocks voltage-gated potassium channels, thus inhibiting neuronal repolarization.
HsTx1 is a toxin from the venom of the scorpion Heterometrus spinifer. HsTx1 is a very potent inhibitor of the rat Kv1.3 voltage-gated potassium channel.
HgeTx1 (systematic name: α-KTx 6.14) is a toxin produced by the Mexican scorpion Hoffmanihadrurus gertschi that is a reversible blocker of the Shaker B K+-channel, a type of voltage-gated potassium channels.
Pi4 is a short toxin from the scorpion Pandinus imperator that blocks specific potassium channels.
BmP02, also known as α-KTx 9.1 or Bmkk(6), is a toxin from the Buthus Martensi Karsch (BmK) scorpion. The toxin acts on potassium channels, blocking Kv1.3 and slowing the deactivation of Kv4.2. BmP02 is not toxic to humans or mice.

Ctri9577 (α-KTx15.10) is a neurotoxin present in the venom of the Chaerilus tricostatus scorpion. This voltage-gated potassium channel specific toxin operates as a potent blocker for Kv1.3 channels as well as a gating modifier of Kv4.3 channels.
κ-KTx2.5 is a toxin found in the venom of the scorpion, Opisthacanthuscayaporum. The toxin belongs to the κ-KTx family, a channel blocker family that targets voltage-gated potassium channels (Kv) 1.1 and 1.4.














