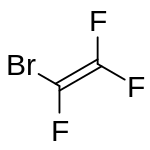
 | |
| Names | |
|---|---|
| IUPAC name bromotrifluoroethene | |
| Other names trifluorovinyl bromide, trifluorobromoethylene | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.009.045 |
| EC Number |
|
PubChem CID | |
| UN number | 2419 |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C2BrF3 | |
| Molar mass | 160.921 g·mol−1 |
| Appearance | colourless gas |
| Odor | moldy, [1] phosgene-like [2] |
| Boiling point | –2.5°C [2] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | spontaneous polymerisation, flammable [3] |
| GHS labelling: | |
    | |
| Danger | |
| H220, H280, H315, H319, H330, H332, H335 | |
| P203, P210, P222, P260, P261, P264, P264+P265, P271, P280, P284, P302+P352, P304+P340, P305+P351+P338, P316, P317, P319, P320, P321, P332+P317, P337+P317, P362+P364, P377, P381, P403, P403+P233, P405, P410+P403, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Bromotrifluoroethylene (BTFE) is a halogenated ethylene derivative with the chemical formula F2CCBrF. It is a highly flammable colourless gas with a musty odour resembling phosgene. It can polymerise spontaneously. [3]