
Gel electrophoresis is a method for separation and analysis of macromolecules and their fragments, based on their size and charge. It is used in clinical chemistry to separate proteins by charge or size and in biochemistry and molecular biology to separate a mixed population of DNA and RNA fragments by length, to estimate the size of DNA and RNA fragments or to separate proteins by charge.

Titration is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte. A reagent, termed the titrant or titrator, is prepared as a standard solution of known concentration and volume. The titrant reacts with a solution of analyte to determine the analyte's concentration. The volume of titrant that reacted with the analyte is termed the titration volume.
A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. In nature, there are many systems that use buffering for pH regulation. For example, the bicarbonate buffering system is used to regulate the pH of blood, and bicarbonate also acts as a buffer in the ocean.

Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms.
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction

Histidine (symbol His or H) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the deprotonated –COO− form under biological conditions), and an imidazole side chain (which is partially protonated), classifying it as a positively charged amino acid at physiological pH. Initially thought essential only for infants, it has now been shown in longer-term studies to be essential for adults also. It is encoded by the codons CAU and CAC.

Boric acid, also called hydrogen borate, boracic acid, and orthoboric acid is a weak, monobasic Lewis acid of boron. However, some of its behaviour towards some chemical reactions suggest it to be tribasic acid in the Brønsted sense as well. Boric acid is often used as an antiseptic, insecticide, flame retardant, neutron absorber, or precursor to other chemical compounds. It has the chemical formula H3BO3 (sometimes written B(OH)3), and exists in the form of colorless crystals or a white powder that dissolves in water. When occurring as a mineral, it is called sassolite.

Borax, also known as sodium borate, sodium borate decahydrate or sodium tetraborate decahydrate, is a hydrate salt of boric acid. Commonly available in powder or granular form, it dissolves in water to make a basic, aqueous solution. It is soluble and has many industrial and household applications as a component in a wide range of products. Applications include as a pesticide; metal soldering; glaze and enamel manufacturing; tanning of skins and hides; artificial aging of wood; as a preservative against wood fungus; analytical chemistry as a buffering agent; and pharmaceutic aid as an alkalizer.

Polyacrylamide gel electrophoresis (PAGE) is a technique widely used in biochemistry, forensic chemistry, genetics, molecular biology and biotechnology to separate biological macromolecules, usually proteins or nucleic acids, according to their electrophoretic mobility. Electrophoretic mobility is a function of the length, conformation and charge of the molecule. Polyacrylamide gel electrophoresis is a powerful tool used to analyze RNA samples. When polyacrylamide gel is denatured after electrophoresis, it provides information on the sample composition of the RNA species.
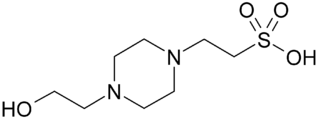
HEPES is a zwitterionic sulfonic acid buffering agent; one of the twenty Good's buffers. HEPES is widely used in cell culture, largely because it is better at maintaining physiological pH despite changes in carbon dioxide concentration when compared to bicarbonate buffers, which are also commonly used in cell culture. Lepe-Zuniga et al. reported an unwanted photochemical process wherein HEPES when exposed to ambient light produces hydrogen peroxide, which is not a problem in bicarbonate-based cell culture buffers. It is therefore strongly advised to keep HEPES-containing solutions in darkness as much as possible to prevent oxidation.
Caps is the plural form of headgear cap.
In organic chemistry, a carbodiimide is a functional group with the formula RN=C=NR. They are exclusively synthetic. A well known carbodiimide is dicyclohexylcarbodiimide, which is used in peptide synthesis. Dialkylcarbodiimides are stable. Some diaryl derivatives tend to convert to dimers and polymers upon standing at room temperature, though this mostly occurs with low melting point carbodiimides that are liquids at room temperature. Solid diaryl carbodiimides are more stable, but can slowly undergo hydrolysis in the presence of water over time.

Bis-tris propane, or 1,3-bis(tris methylamino)propane, also known as BTP, is a chemical substance that is used in buffer solutions. It is a white to off-white crystalline powder that is soluble in water. It has a wide buffering range, from 6 to 9.5 due to its two pKa values which are close in value. This buffer is primarily used in biochemistry and molecular biology.
Good's buffers are twenty buffering agents for biochemical and biological research selected and described by Norman Good and colleagues during 1966–1980. Most of the buffers were new zwitterionic compounds prepared and tested by Good and coworkers for the first time, though some were known compounds previously overlooked by biologists. Before Good's work, few hydrogen ion buffers between pH 6 and 8 had been accessible to biologists, and very inappropriate, toxic, reactive and inefficient buffers had often been used. Many Good's buffers became and remain crucial tools in modern biological laboratories.

PIPES is the common name for piperazine-N,N′-bis(2-ethanesulfonic acid), and is a frequently used buffering agent in biochemistry. It is an ethanesulfonic acid buffer developed by Good et al. in the 1960s.

MOPS is a buffer introduced by Good et al. in the 1960s. It is a structural analog to MES. Its chemical structure contains a morpholine ring. HEPES is a similar pH buffering compound that contains a piperazine ring. With a pKa of 7.20, MOPS is an excellent buffer for many biological systems at near-neutral pH.
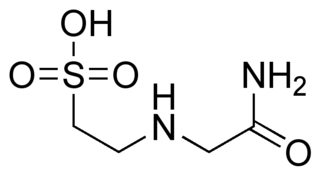
ACES is the common abbreviation for the compound N-(2-Acetamido)-2-aminoethanesulfonic acid.

MES is the common name for the compound 2-(N-morpholino)ethanesulfonic acid. Its chemical structure contains a morpholine ring. It has a molecular weight of 195.2 and the chemical formula is C6H13NO4S. Synonyms include: 2-morpholinoethanesulfonic acid; 2-(4-morpholino)ethanesulfonic acid; 2-(N-morpholino)ethanesulfonic acid; 2-(4-morpholino)ethanesulfonic acid; MES; MES hydrate; and morpholine-4-ethanesulfonic acid hydrate. MOPS is a similar pH buffering compound which contains a propanesulfonic moiety instead of an ethanesulfonic one.
HEPPS (EPPS) is a buffering agent used in biology and biochemistry. The pKa of HEPPS is 8.00. It is ones of Good's buffers.

Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs.















