In chemistry, a zwitterion, also called an inner salt or dipolar ion, is a molecule that contains an equal number of positively- and negatively-charged functional groups. With amino acids, for example, in solution a chemical equilibrium will be established between the "parent" molecule and the zwitterion.

A detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. There are a large variety of detergents, a common family being the alkylbenzene sulfonates, which are soap-like compounds that are more soluble in hard water, because the polar sulfonate is less likely than the polar carboxylate to bind to calcium and other ions found in hard water.

Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. Surfactants may function as emulsifiers, wetting agents, detergents, foaming agents, or dispersants. The word "surfactant" is a blend of surface-active agent, coined c. 1950.
Lysis is the breaking down of the membrane of a cell, often by viral, enzymic, or osmotic mechanisms that compromise its integrity. A fluid containing the contents of lysed cells is called a lysate. In molecular biology, biochemistry, and cell biology laboratories, cell cultures may be subjected to lysis in the process of purifying their components, as in protein purification, DNA extraction, RNA extraction, or in purifying organelles.
A lysis buffer is a buffer solution used for the purpose of breaking open cells for use in molecular biology experiments that analyze the labile macromolecules of the cells. Most lysis buffers contain buffering salts and ionic salts to regulate the pH and osmolarity of the lysate. Sometimes detergents are added to break up membrane structures. For lysis buffers targeted at protein extraction, protease inhibitors are often included, and in difficult cases may be almost required. Lysis buffers can be used on both animal and plant tissue cells.

Phosphatidylcholines (PC) are a class of phospholipids that incorporate choline as a headgroup. They are a major component of biological membranes and can be easily obtained from a variety of readily available sources, such as egg yolk or soybeans, from which they are mechanically or chemically extracted using hexane. They are also a member of the lecithin group of yellow-brownish fatty substances occurring in animal and plant tissues. Dipalmitoylphosphatidylcholine (lecithin) is a major component of the pulmonary surfactant, and is often used in the lecithin–sphingomyelin ratio to calculate fetal lung maturity. While phosphatidylcholines are found in all plant and animal cells, they are absent in the membranes of most bacteria, including Escherichia coli. Purified phosphatidylcholine is produced commercially.
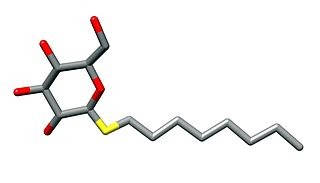
n-Octyl β-d-thioglucopyranoside is a mild nonionic detergent that is used for cell lysis or to solubilise membrane proteins without denaturing them. This is particularly of use in order to crystallise them or to reconstitute them into lipid bilayers. It has a critical micelle concentration of 9 mM.

The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and exclude water molecules. The word hydrophobic literally means "water-fearing", and it describes the segregation of water and nonpolar substances, which maximizes hydrogen bonding between molecules of water and minimizes the area of contact between water and nonpolar molecules. In terms of thermodynamics, the hydrophobic effect is the free energy change of water surrounding a solute. A positive free energy change of the surrounding solvent indicates hydrophobicity, whereas a negative free energy change implies hydrophilicity.
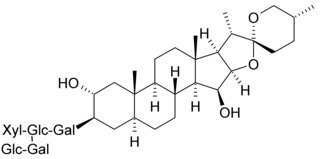
Digitonin is a steroidal saponin (saraponin) obtained from the foxglove plant Digitalis purpurea. Its aglycone is digitogenin, a spirostan steroid. It has been investigated as a detergent, as it effectively water-solubilizes lipids. As such, it has several potential membrane-related applications in biochemistry, including solubilizing membrane proteins, precipitating cholesterol, and permeabilizing cell membranes.

An amphiphile, or amphipath, is a chemical compound possessing both hydrophilic and lipophilic (fat-loving) properties. Such a compound is called amphiphilic or amphipathic. Amphiphilic compounds include surfactants. The phospholipid amphiphiles are the major structural component of cell membranes.

Triton X-100 is a nonionic surfactant that has a hydrophilic polyethylene oxide chain and an aromatic hydrocarbon lipophilic or hydrophobic group. The hydrocarbon group is a 4-(1,1,3,3-tetramethylbutyl)-phenyl group. Triton X-100 is closely related to IGEPAL CA-630, which might differ from it mainly in having slightly shorter ethylene oxide chains. As a result, Triton X-100 is slightly more hydrophilic than Igepal CA-630 thus these two detergents may not be considered to be functionally interchangeable for most applications.

Surfactin is a cyclic lipopeptide, commonly used as an antibiotic for its capacity as a surfactant. It is an amphiphile capable of withstanding hydrophilic and hydrophobic environments. The Gram-positive bacterial species Bacillus subtilis produces surfactin for its antibiotic effects against competitors. Surfactin showcases antibacterial, antiviral, antifungal, and hemolytic effects.
Cleavable detergents, also known as cleavable surfactants, are special surfactants (detergents) that are used in biochemistry and especially in proteomics to enhance protein denaturation and solubility. The detergent is rendered inactive by cleavage, usually under acidic conditions, in order to make the sample compatible with a following procedure or in order to selectively remove the cleavage products.
1,3-Propane sultone is the organosulfur compound with the formula (CH2)3SO3. It is a cyclic sulfonate ester, a class of compounds called sultones. It is a readily melting colorless solid.
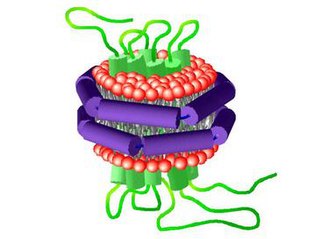
A nanodisc is a synthetic model membrane system which assists in the study of membrane proteins. Nanodiscs are discoidal proteins in which a lipid bilayer is surrounded by molecules that are amphipathic molecules including proteins, peptides, and synthetic polymers. It is composed of a lipid bilayer of phospholipids with the hydrophobic edge screened by two amphipathic proteins. These proteins are called membrane scaffolding proteins (MSP) and align in double belt formation. Nanodiscs are structurally very similar to discoidal high-density lipoproteins (HDL) and the MSPs are modified versions of apolipoprotein A1 (apoA1), the main constituent in HDL. Nanodiscs are useful in the study of membrane proteins because they can solubilise and stabilise membrane proteins and represent a more native environment than liposomes, detergent micelles, bicelles and amphipols.
Octyl glucoside is a nonionic surfactant frequently used to solubilise integral membrane proteins for studies in biochemistry. Structurally, it is a glycoside derived from glucose and octanol. Like Genapol X-100 and Triton X-100, it is a nonphysiological amphiphile that makes lipid bilayers less "stiff".
Hydrophobicity scales are values that define the relative hydrophobicity or hydrophilicity of amino acid residues. The more positive the value, the more hydrophobic are the amino acids located in that region of the protein. These scales are commonly used to predict the transmembrane alpha-helices of membrane proteins. When consecutively measuring amino acids of a protein, changes in value indicate attraction of specific protein regions towards the hydrophobic region inside lipid bilayer.
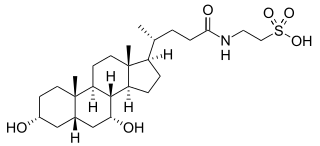
Taurochenodeoxycholic acid is a bile acid formed in the liver of most species, including humans, by conjugation of chenodeoxycholic acid with taurine. It is secreted into bile and then into the intestine. It is usually ionized at physiologic pH. However, although it can be crystallized as the sodium salt.

A maltoside is a glycoside with maltose as the glycone (sugar) functional group. Among the most common are alkyl maltosides, which contain hydrophobic alkyl chains as the aglycone. Given their amphiphilic properties, these comprise a class of detergents, where variation in the alkyl chain confers a range of detergent properties including CMC and solubility. Maltosides are most often used for the solubilization and purification of membrane proteins.
Amphipols are a class of amphiphilic polymers designed to keep membrane proteins soluble in water without the need for detergents, which are traditionally used to this end but tend to be denaturing. Amphipols adsorb onto the hydrophobic transmembrane surface of membrane proteins thanks to their hydrophobic moieties and keep the complexes thus formed water-soluble thanks to the hydrophilic ones. Amphipol-trapped membrane proteins are, as a rule, much more stable than detergent-solubilized ones, which facilitates their study by most biochemical and biophysical approaches. Amphipols can be used to fold denatured membrane proteins to their native form and have proven particularly precious in the field of single-particle electron cryo-microscopy .The properties and uses of amphipols and other non-conventional surfactants are the subject of a book by Jean-Luc Popot.












