
Toll-like receptors (TLRs) are a class of proteins that play a key role in the innate immune system. They are single-spanning receptors usually expressed on sentinel cells such as macrophages and dendritic cells, that recognize structurally conserved molecules derived from microbes. Once these microbes have reached physical barriers such as the skin or intestinal tract mucosa, they are recognized by TLRs, which activate immune cell responses. The TLRs include TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, TLR10, TLR11, TLR12, and TLR13. Humans lack genes for TLR11, TLR12 and TLR13 and mice lack a functional gene for TLR10. The receptors TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are located on the cell membrane, whereas TLR3, TLR7, TLR8, and TLR9 are located in intracellular vesicles.
Pattern recognition receptors (PRRs) play a crucial role in the proper function of the innate immune system. PRRs are germline-encoded host sensors, which detect molecules typical for the pathogens. They are proteins expressed mainly by cells of the innate immune system, such as dendritic cells, macrophages, monocytes, neutrophils, as well as by epithelial cells, to identify two classes of molecules: pathogen-associated molecular patterns (PAMPs), which are associated with microbial pathogens, and damage-associated molecular patterns (DAMPs), which are associated with components of host's cells that are released during cell damage or death. They are also called primitive pattern recognition receptors because they evolved before other parts of the immune system, particularly before adaptive immunity. PRRs also mediate the initiation of antigen-specific adaptive immune response and release of inflammatory cytokines.

The innate, or nonspecific, immune system is one of the two main immunity strategies in vertebrates. The innate immune system is an alternate defense strategy and is the dominant immune system response found in plants, fungi, insects, and primitive multicellular organisms.

The lectin pathway or MBL pathway is a type of cascade reaction in the complement system, similar in structure to the classical complement pathway, in that, after activation, it proceeds through the action of C4 and C2 to produce activated complement proteins further down the cascade. In contrast to the classical complement pathway, the lectin pathway does not recognize an antibody bound to its target. The lectin pathway starts with mannose-binding lectin (MBL) or ficolin binding to certain sugars.
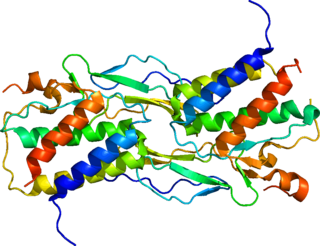
Interleukin-15 (IL-15) is a protein that in humans is encoded by the IL15 gene. IL-15 is an inflammatory cytokine with structural similarity to Interleukin-2 (IL-2). Like IL-2, IL-15 binds to and signals through a complex composed of IL-2/IL-15 receptor beta chain (CD122) and the common gamma chain. IL-15 is secreted by mononuclear phagocytes following infection by virus(es). This cytokine induces the proliferation of natural killer cells, i.e. cells of the innate immune system whose principal role is to kill virally infected cells.

Interleukin 24 (IL-24) is a protein in the interleukin family, a type of cytokine signaling molecule in the immune system. In humans, this protein is encoded by the IL24 gene.

Interleukin-22 (IL-22) is protein that in humans is encoded by the IL22 gene.
Collectins (collagen-containing C-type lectins) are a part of the innate immune system. They form a family of collagenous Ca2+-dependent defense lectins, which are found in animals. Collectins are soluble pattern recognition receptors (PRRs). Their function is to bind to oligosaccharide structure or lipids that are on the surface of microorganisms. Like other PRRs they bind pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) of oligosaccharide origin. Binding of collectins to microorganisms may trigger elimination of microorganisms by aggregation, complement activation, opsonization, activation of phagocytosis, or inhibition of microbial growth. Other functions of collectins are modulation of inflammatory, allergic responses, adaptive immune system and clearance of apoptotic cells.
The mannose receptor is a C-type lectin primarily present on the surface of macrophages, immature dendritic cells and liver sinusoidal endothelial cells, but is also expressed on the surface of skin cells such as human dermal fibroblasts and keratinocytes. It is the first member of a family of endocytic receptors that includes Endo180 (CD280), M-type PLA2R, and DEC-205 (CD205).

Toll-like receptor 6 is a protein that in humans is encoded by the TLR6 gene. TLR6 is a transmembrane protein, member of toll-like receptor family, which belongs to the pattern recognition receptor (PRR) family. TLR6 acts in a heterodimer form with toll-like receptor 2 (TLR2). Its ligands include multiple diacyl lipopeptides derived from gram-positive bacteria and mycoplasma and several fungal cell wall saccharides. After dimerizing with TLR2, the NF-κB intracellular signalling pathway is activated, leading to a pro-inflammatory cytokine production and activation of innate immune response. TLR6 has also been designated as CD286.

C-type lectin domain family 7 member A or Dectin-1 is a protein that in humans is encoded by the CLEC7A gene. CLEC7A is a member of the C-type lectin/C-type lectin-like domain (CTL/CTLD) superfamily. The encoded glycoprotein is a small type II membrane receptor with an extracellular C-type lectin-like domain fold and a cytoplasmic domain with a partial immunoreceptor tyrosine-based activation motif. It functions as a pattern-recognition receptor for a variety of β-1,3-linked and β-1,6-linked glucans from fungi and plants, and in this way plays a role in innate immune response. Expression is found on myeloid dendritic cells, monocytes, macrophages and B cells. Alternate transcriptional splice variants, encoding different isoforms, have been characterized. This gene is closely linked to other CTL/CTLD superfamily members on chromosome 12p13 in the natural killer gene complex region.
Interleukin-28 receptor is a type II cytokine receptor found largely in epithelial cells. It binds type 3 interferons, interleukin-28 A, Interleukin-28B, interleukin 29 and interferon lambda 4. It consists of an α chain and shares a common β subunit with the interleukin-10 receptor. Binding to the interleukin-28 receptor, which is restricted to select cell types, is important for fighting infection. Binding of the type 3 interferons to the receptor results in activation of the JAK/STAT signaling pathway.

Caspase recruitment domain-containing protein 9 is an adaptor protein of the CARD-CC protein family, which in humans is encoded by the CARD9 gene. It mediates signals from pattern recognition receptors to activate pro-inflammatory and anti-inflammatory cytokines, regulating inflammation. Homozygous mutations in CARD9 are associated with defective innate immunity against yeasts, like Candida and dermatophytes.
The following outline is provided as an overview of and topical guide to immunology:

C-type lectin domain family 5 member A (CLEC5A), also known as C-type lectin superfamily member 5 (CLECSF5) and myeloid DAP12-associating lectin 1 (MDL-1) is a C-type lectin that in humans is encoded by the CLEC5A gene.

NKG2D is an activating receptor (transmembrane protein) belonging to the NKG2 family of C-type lectin-like receptors. NKG2D is encoded by KLRK1 (killer cell lectin like receptor K1) gene which is located in the NK-gene complex (NKC) situated on chromosome 6 in mice and chromosome 12 in humans. In mice, it is expressed by NK cells, NK1.1+ T cells, γδ T cells, activated CD8+ αβ T cells and activated macrophages. In humans, it is expressed by NK cells, γδ T cells and CD8+ αβ T cells. NKG2D recognizes induced-self proteins from MIC and RAET1/ULBP families which appear on the surface of stressed, malignant transformed, and infected cells.
Immunology is the study of the immune system during health and disease. Below is a list of immunology-related articles.
An interferon-stimulated gene (ISG) is a gene that can be expressed in response to stimulation by interferon. Interferons bind to receptors on the surface of a cell, initiating protein signaling pathways within the cell. This interaction leads to the expression of a subset of genes involved in the innate immune system response. ISGs are commonly expressed in response to viral infection, but also during bacterial infection and in the presence of parasites. It's currently estimated that 10% of the human genome is regulated by interferons (IFNs). Interferon stimulated genes can act as an initial response to pathogen invasion, slowing down viral replication and increasing expression of immune signaling complexes. There are three known types of interferon. With approximately 450 genes highly expressed in response to interferon type I. Type I interferon consists of INF-α, INF-β, INF-ω and is expressed in response to viral infection. ISGs induced by type I interferon are associated with viral replication suppression and increase expression of immune signaling proteins. Type II interferon consists only of INF-γ and is associated with controlling intracellular pathogens and tumor suppressor genes. Type III interferon consists of INF-λ and is associated with viral immune response and is key in anti-fungal neutrophil response.
Candidalysin is a cytolytic 31-amino acid α-helical amphipathic peptide toxin secreted by the opportunistic pathogen Candida albicans. This toxin is a fungal example of a classical virulence factor. Hyphal morphogenesis in C. albicans is associated with damage to host epithelial cells; during this process Candidalysin is released and intercalates in host membranes. Candidalysin promotes damage of oral epithelial cells and induces lactate dehydrogenase release and calcium ion influx. It is unique in the fact that it is the first peptide toxin to be identified in any human fungal pathogen.

Mihai G. Netea is a Romanian Dutch physician and professor at Radboud University Nijmegen, specialized in infectious disease, immunology, and global health.















