
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid, AKA phosphoric acid H3PO4.
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.

In chemistry, pyrophosphates are phosphorus oxyanions that contain two phosphorus atoms in a P–O–P linkage. A number of pyrophosphate salts exist, such as disodium pyrophosphate (Na2H2P2O7) and tetrasodium pyrophosphate (Na4P2O7), among others. Often pyrophosphates are called diphosphates. The parent pyrophosphates are derived from partial or complete neutralization of pyrophosphoric acid. The pyrophosphate bond is also sometimes referred to as a phosphoanhydride bond, a naming convention which emphasizes the loss of water that occurs when two phosphates form a new P–O–P bond, and which mirrors the nomenclature for anhydrides of carboxylic acids. Pyrophosphates are found in ATP and other nucleotide triphosphates, which are important in biochemistry. The term pyrophosphate is also the name of esters formed by the condensation of a phosphorylated biological compound with inorganic phosphate, as for dimethylallyl pyrophosphate. This bond is also referred to as a high-energy phosphate bond.

Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of OH−, F− and Cl− ion, respectively, in the crystal. The formula of the admixture of the three most common endmembers is written as Ca10(PO4)6(OH,F,Cl)2, and the crystal unit cell formulae of the individual minerals are written as Ca10(PO4)6(OH)2, Ca10(PO4)6F2 and Ca10(PO4)6Cl2.
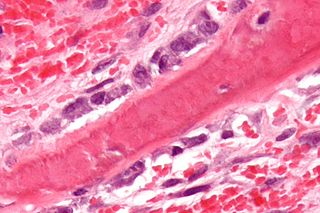
Osteoblasts are cells with a single nucleus that synthesize bone. However, in the process of bone formation, osteoblasts function in groups of connected cells. Individual cells cannot make bone. A group of organized osteoblasts together with the bone made by a unit of cells is usually called the osteon.

Ultrastructure is the architecture of cells and biomaterials that is visible at higher magnifications than found on a standard optical light microscope. This traditionally meant the resolution and magnification range of a conventional transmission electron microscope (TEM) when viewing biological specimens such as cells, tissue, or organs. Ultrastructure can also be viewed with scanning electron microscopy and super-resolution microscopy, although TEM is a standard histology technique for viewing ultrastructure. Such cellular structures as organelles, which allow the cell to function properly within its specified environment, can be examined at the ultrastructural level.
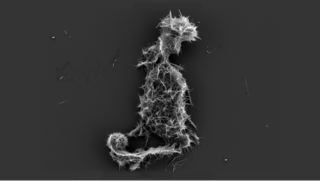
The term calcium phosphate refers to a family of materials and minerals containing calcium ions (Ca2+) together with inorganic phosphate anions. Some so-called calcium phosphates contain oxide and hydroxide as well. Calcium phosphates are white solids of nutritional value and are found in many living organisms, e.g., bone mineral and tooth enamel. In milk, it exists in a colloidal form in micelles bound to casein protein with magnesium, zinc, and citrate–collectively referred to as colloidal calcium phosphate (CCP). Various calcium phosphate minerals are used in the production of phosphoric acid and fertilizers. Overuse of certain forms of calcium phosphate can lead to nutrient-containing surface runoff and subsequent adverse effects upon receiving waters such as algal blooms and eutrophication (over-enrichment with nutrients and minerals).

Hydroxyapatite is a naturally occurring mineral form of calcium apatite with the formula Ca5(PO4)3(OH), often written Ca10(PO4)6(OH)2 to denote that the crystal unit cell comprises two entities. It is the hydroxyl endmember of the complex apatite group. The OH− ion can be replaced by fluoride or chloride, producing fluorapatite or chlorapatite. It crystallizes in the hexagonal crystal system. Pure hydroxyapatite powder is white. Naturally occurring apatites can, however, also have brown, yellow, or green colorations, comparable to the discolorations of dental fluorosis.

Tricalcium phosphate (sometimes abbreviated TCP), more commonly known as Calcium phosphate, is a calcium salt of phosphoric acid with the chemical formula Ca3(PO4)2. It is also known as tribasic calcium phosphate and bone phosphate of lime (BPL). It is a white solid of low solubility. Most commercial samples of "tricalcium phosphate" are in fact hydroxyapatite.

Corpora arenacea are calcified structures in the pineal gland and other areas of the brain such as the choroid plexus. Older organisms have numerous corpora arenacea, whose function, if any, is unknown. Concentrations of "brain sand" increase with age, so the pineal gland becomes increasingly visible on X-rays over time, usually by the third or fourth decade. They are sometimes used as anatomical landmarks in radiological examinations.
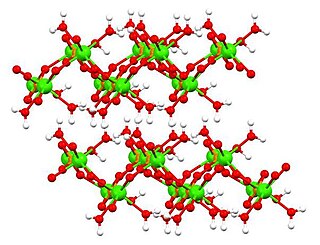
Dicalcium phosphate is the calcium phosphate with the formula CaHPO4 and its dihydrate. The "di" prefix in the common name arises because the formation of the HPO42– anion involves the removal of two protons from phosphoric acid, H3PO4. It is also known as dibasic calcium phosphate or calcium monohydrogen phosphate. Dicalcium phosphate is used as a food additive, it is found in some toothpastes as a polishing agent and is a biomaterial.
In pharmacology, biological activity or pharmacological activity describes the beneficial or adverse effects of a drug on living matter. When a drug is a complex chemical mixture, this activity is exerted by the substance's active ingredient or pharmacophore but can be modified by the other constituents. Among the various properties of chemical compounds, pharmacological/biological activity plays a crucial role since it suggests uses of the compounds in the medical applications. However, chemical compounds may show some adverse and toxic effects which may prevent their use in medical practice.

Statherin is a protein in humans that is encoded by the STATH gene. It prevents the precipitation of calcium phosphate in saliva, maintaining a high calcium level in saliva available for remineralisation of tooth enamel and high phosphate levels for buffering.
Amorphous calcium phosphate (ACP) is a glassy solid that is formed from the chemical decomposition of a mixture of dissolved phosphate and calcium salts (e.g. (NH4)2HPO4 + Ca(NO3)2). The resulting amorphous mixture consists mostly of calcium and phosphate, but also contains varying amounts of water and hydrogen and hydroxide ions, depending on the synthesis conditions. Such mixtures are also known as calcium phosphate cement.
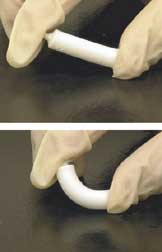
Artificial bone refers to bone-like material created in a laboratory that can be used in bone grafts, to replace human bone that was lost due to severe fractures, disease, etc.
Octacalcium phosphate (sometimes referred to as OCP) is a form of calcium phosphate with formula Ca8H2(PO4)6·5H2O. OCP may be a precursor to tooth enamel, dentine, and bones. OCP is a precursor of hydroxyapatite (HA), an inorganic biomineral that is important in bone growth. OCP has garnered lots of attention due to its inherent biocompatibility. While OCP exhibits good properties in terms of bone growth, very stringent synthesis requirements make it difficult for mass productions, but nevertheless has shown promise not only in-vitro, but also in in-vivo clinical case studies.
Milwaukee shoulder syndrome (MSS) (apatite-associated destructive arthritis/Basic calcium phosphate (BCP) crystal arthritis/rapid destructive arthritis of the shoulder is a rare rheumatological condition similar to pseudogout, associated with periarticular or intra-articular deposition of hydroxyapatite or basic calcium phosphate (BCP) crystals. While primarily associated with the shoulder joint, it can affect any joint in the body below the head. Along with symptomatology, the disease typically presents with positive radiologic findings, often showing marked erosion of the humeral head, cartilage, capsule, and bursae. Though rare, it is most often seen in females beginning in their 50s or 60s. Patients often have a history of joint trauma or overuse, calcium pyrophosphate dehydrate crystal deposition, neuroarthropathy, dialysis-related arthropathy or denervation.
A simulated body fluid (SBF) is a solution with an ion concentration close to that of human blood plasma, kept under mild conditions of pH and identical physiological temperature. SBF was first introduced by Kokubo et al. in order to evaluate the changes on a surface of a bioactive glass ceramic. Later, cell culture media, in combination with some methodologies adopted in cell culture, were proposed as an alternative to conventional SBF in assessing the bioactivity of materials.
Tetracalcium phosphate is the compound Ca4(PO4)2O, (4CaO·P2O5). It is the most basic of the calcium phosphates, and has a Ca/P ratio of 2, making it the most phosphorus poor phosphate. It is found as the mineral hilgenstockite, which is formed in industrial phosphate rich slag (called "Thomas slag"). This slag was used as a fertiliser due to the higher solubility of tetracalcium phosphate relative to apatite minerals. Tetracalcium phosphate is a component in some calcium phosphate cements that have medical applications.
Defluoridation is the downward adjustment of the level of fluoride in drinking water. Worldwide, fluoride is one of the most abundant anions present in groundwater. Fluoride is more present in groundwater than surface water mainly due to the leaching of minerals. Groundwater accounts for 98 percent of the earth's potable water. An excess of fluoride in drinking water causes dental fluorosis and skeletal fluorosis. The World Health Organization has recommended a guideline value of 1.5 mg/L as the concentration above which dental fluorosis is likely. Fluorosis is endemic in more than 20 developed and developing nations.












