
Campylobacter is a type of bacteria that can cause a diarrhea disease in people. Its name means "curved bacteria", as the germ typically appears in a comma or "s" shape. According to its scientific classification, it is a genus of gram-negative bacteria that is motile.

Fusobacterium is a genus of obligate anaerobic, Gram-negative, non-sporeforming bacteria belonging to Gracilicutes. Individual cells are slender, rod-shaped bacilli with pointed ends. Fusobacterium was discovered in 1900 by Courmont and Cade and is common in the flora of humans.

Helicobacter is a genus of gram-negative bacteria possessing a characteristic helical shape. They were initially considered to be members of the genus Campylobacter, but in 1989, Goodwin et al. published sufficient reasons to justify the new genus name Helicobacter. The genus Helicobacter contains about 35 species.

Clostridium perfringens is a Gram-positive, bacillus (rod-shaped), anaerobic, spore-forming pathogenic bacterium of the genus Clostridium. C. perfringens is ever-present in nature and can be found as a normal component of decaying vegetation, marine sediment, the intestinal tract of humans and other vertebrates, insects, and soil. It has the shortest reported generation time of any organism at 6.3 minutes in thioglycolate medium.
Enteritis is inflammation of the small intestine. It is most commonly caused by food or drink contaminated with pathogenic microbes, such as Serratia, but may have other causes such as NSAIDs, radiation therapy as well as autoimmune conditions like Crohn's disease and celiac disease. Symptoms include abdominal pain, cramping, diarrhea, dehydration, and fever. Related diseases of the gastrointestinal system include inflammation of the stomach and large intestine.

Campylobacter jejuni is a species of pathogenic bacteria, one of the most common causes of food poisoning in Europe and in the US. The vast majority of cases occur as isolated events, not as part of recognized outbreaks. Active surveillance through the Foodborne Diseases Active Surveillance Network (FoodNet) indicates that about 20 cases are diagnosed each year for each 100,000 people in the US, while many more cases are undiagnosed or unreported; the CDC estimates a total of 1.5 million infections every year. The European Food Safety Authority reported 246,571 cases in 2018, and estimated approximately nine million cases of human campylobacteriosis per year in the European Union.
Mycobacterium avium subspecies paratuberculosis (MAP) is an obligate pathogenic bacterium in the genus Mycobacterium. It is often abbreviated M. paratuberculosis or M. avium ssp. paratuberculosis. It is the causative agent of Johne's disease, which affects ruminants such as cattle, and suspected causative agent in human Crohn's disease and rheumatoid arthritis. The type strain is ATCC 19698.
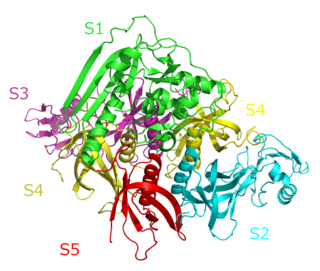
Pertussis toxin (PT) is a protein-based AB5-type exotoxin produced by the bacterium Bordetella pertussis, which causes whooping cough. PT is involved in the colonization of the respiratory tract and the establishment of infection. Research suggests PT may have a therapeutic role in treating a number of common human ailments, including hypertension, viral infection, and autoimmunity.

Shigella flexneri is a species of Gram-negative bacteria in the genus Shigella that can cause diarrhea in humans. Several different serogroups of Shigella are described; S. flexneri belongs to group B. S. flexneri infections can usually be treated with antibiotics, although some strains have become resistant. Less severe cases are not usually treated because they become more resistant in the future. Shigella are closely related to Escherichia coli, but can be differentiated from E.coli based on pathogenicity, physiology and serology.

Campylobacterota are a phylum of bacteria. All species of this phylum are Gram-negative.
Pseudomonas amygdali is a Gram-negative plant pathogenic bacterium. It is named after its ability to cause disease on almond trees. Different analyses, including 16S rRNA analysis, DNA-DNA hybridization, and MLST clearly placed P. amygdali in the P. syringae group together with the species Pseudomonas ficuserectae and Pseudomonas meliae, and 27 pathovars of Pseudomonas syringae/Pseudomonas savastanoi, constituting a single, well-defined phylogenetic group which should be considered as a single species. This phylogenetic group has not been formally named because of the lack of reliable means to differentiate it phenotypically from closely related species, and it is currently known as either genomospecies 2 or phylogroup 3. When it is formally named, the correct name for this new species should be Pseudomonas amygdali, which takes precedence over all the other names of taxa from this group, including Pseudomonas savastanoi, which is and inadequate and confusing name whose use is not recommended.

Cronobacter sakazakii, which before 2007 was named Enterobacter sakazakii, is an opportunistic Gram-negative, rod-shaped, pathogenic bacterium that can live in very dry places, otherwise known as xerotolerance. C. sakazakii utilizes a number of genes to survive desiccation and this xerotolerance may be strain specific. The majority of C. sakazakii cases are adults but low-birth-weight preterm neonatal and older infants are at the highest risk. The pathogen is a rare cause of invasive infection in infants, with historically high case fatality rates (40–80%).
Campylobacter upsaliensis is a gram-negative bacteria in the Campylobacter genus. C. upsaliensis is found worldwide, and is a common cause of campylobacteriosis in humans, as well as gastroenteritis in dogs. Human infections are primarily associated with raw or undercooked meat and contaminated water sources, however there is some zoonotic risk associated with the spread from dogs. C. upsaliensis primarily affects the gastrointestinal tract as it damages gastrointestinal epithelial cells. There are many methods for detecting C.upsaliensis including PCR and ELISA, however there is no current gold standard in detection techniques. Infection is typically self limiting, however there is antimicrobial therapy available.
Faecalibacterium is a genus of bacteria. The genus contains several species including Faecalibacterium prausnitzii, Faecalibacterium butyricigenerans, Faecalibacterium longum, Faecalibacterium duncaniae, Faecalibacterium hattorii, and Faecalibacterium gallinarum. Its first known species, Faecalibacterium prausnitzii is gram-positive, mesophilic, rod-shaped, and anaerobic, and is one of the most abundant and important commensal bacteria of the human gut microbiota. It is non-spore forming and non-motile. These bacteria produce butyrate and other short-chain fatty acids through the fermentation of dietary fiber. The production of butyrate makes them an important member of the gut microbiota, fighting against inflammation.
Arcobacter is a genus of Gram-negative, spiral-shaped bacteria in the phylum Campylobacterota. It shows an unusually wide range of habitats, and some species can be human and animal pathogens. Species of the genus Arcobacter are found in both animal and environmental sources, making it unique among the Campylobacterota. This genus currently consists of five species: A. butzleri, A. cryaerophilus, A. skirrowii, A. nitrofigilis, and A. sulfidicus, although several other potential novel species have recently been described from varying environments. Three of these five known species are pathogenic. Members of this genus were first isolated in 1977 from aborted bovine fetuses. They are aerotolerant, Campylobacter-like organisms, previously classified as Campylobacter. The genus Arcobacter, in fact, was created as recently as 1992. Although they are similar to this other genus, Arcobacter species can grow at lower temperatures than Campylobacter, as well as in the air, which Campylobacter cannot.
Campylobacter mucosalis was initially isolated in 1974 by Lawson and Rowland from the lesions of porcine intestinal adenomatosis. Isolated species were gram-negative, microaerophilic and curve shaped. These organisms resembled Campylobacter sputorum in their morphological and phenotypic characteristics and were given the name Campylobacter sputorum subsp. mucosalis. A study, using DNA homology experiments, found that Campylobacter sputorum subsp. mucosalis is a distinct species and is not a subspecies of C. sputorum. Thus, its name was changed to Campylobacter mucosalis.
Cronobacter dublinensis is a bacterium. Its name pertains to Dublin, the origin of the type strain. The type strain is originally from a milk powder manufacturing facility. C. dublinensis sp. nov. is dulcitol negative and methyl-α-D-glucopyranoside positive and generally positive for indole production.
Campylobacter showae is a species of Campylobacter found in humans. It is gram-negative, straight rod-shaped, motile by means of multiple unipolar flagella. It is asaccharolytic and prefers an anaerobic atmosphere. SU A4 is its type strain. Its genome has been sequenced.
The genus Wolinella is a member of the Campylobacterales order of Bacteria. The order Campylobacterales includes human pathogens such as Helicobacter pylori and Campylobacter jejuni.
Vaccine resistance is the evolutionary adaptation of pathogens to infect and spread through vaccinated individuals, analogous to antimicrobial resistance. It concerns both human and animal vaccines. Although the emergence of a number of vaccine resistant pathogens has been well documented, this phenomenon is nevertheless much more rare and less of a concern than antimicrobial resistance.






