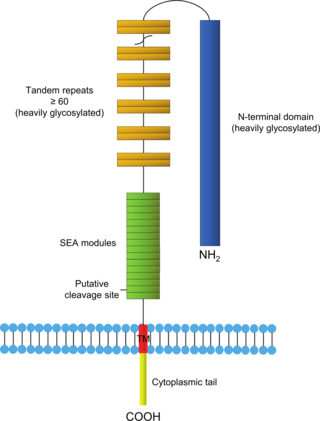
Mucin-16(MUC-16) also known as Ovarian cancer-related tumor marker CA125 is a protein that in humans is encoded by the MUC16 gene. MUC-16 is a member of the mucin family glycoproteins. MUC-16 has found application as a tumor marker or biomarker that may be elevated in the blood of some patients with specific types of cancers, most notably ovarian cancer, or other conditions that are benign.

Human homeostatic iron regulator protein, also known as the HFE protein, is a transmembrane protein that in humans is encoded by the HFE gene. The HFE gene is located on short arm of chromosome 6 at location 6p22.2

Insulin-like growth factor 2 receptor (IGF2R), also called the cation-independent mannose-6-phosphate receptor (CI-MPR) is a protein that in humans is encoded by the IGF2R gene. IGF2R is a multifunctional protein receptor that binds insulin-like growth factor 2 (IGF2) at the cell surface and mannose-6-phosphate (M6P)-tagged proteins in the trans-Golgi network.
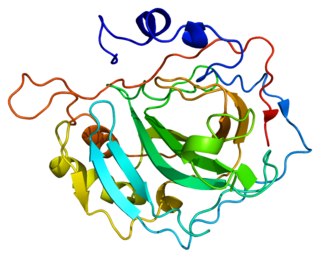
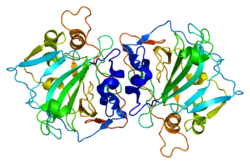
Carbonic anhydrase II is one of sixteen forms of human α carbonic anhydrases. Carbonic anhydrase catalyzes reversible hydration of carbon dioxide. Defects in this enzyme are associated with osteopetrosis and renal tubular acidosis. Renal carbonic anhydrase allows the reabsorption of bicarbonate ions in the proximal tubule. Loss of carbonic anhydrase activity in bones impairs the ability of osteoclasts to promote bone resorption, leading to osteopetrosis.

Carbonic anhydrase 3 is an enzyme that in humans is encoded by the CA3 gene.
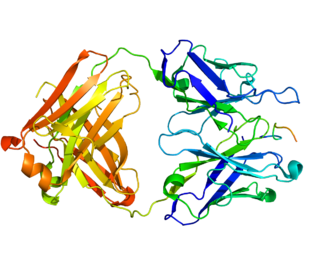
Carbonic anhydrase IX is an enzyme that in humans is encoded by the CA9 gene. It is one of the 14 carbonic anhydrase isoforms found in humans and is a transmembrane dimeric metalloenzyme with an extracellular active site that facilitates acid secretion in the gastrointestinal tract. CA IX is overexpressed in many types of cancer including clear cell renal cell carcinoma (RCC) as well as carcinomas of the cervix, breast and lung where it promotes tumor growth by enhancing tumor acidosis.

Carbonic anhydrase 1 is an enzyme that in humans is encoded by the CA1 gene.

Carbonic anhydrase 4 is an enzyme that in humans is encoded by the CA4 gene.

Carbonic anhydrase 6 is an enzyme that in humans is encoded by the CA6 gene. It is also called 'gustin' because of its presence in saliva, and lower-than-normal levels of salivary zinc in individuals with hypogeusia.

Tumor necrosis factor receptor superfamily member 12A also known as the TWEAK receptor (TWEAKR) is a protein that in humans is encoded by the TNFRSF12A gene.

Anion exchange protein 3 is a membrane transport protein that in humans is encoded by the SLC4A3 gene. AE3 is functionally similar to the Band 3 Cl−/HCO3− exchange protein but it is expressed primarily in brain neurons and in the heart. Like AE2 its activity is sensitive to pH. AE3 mutations have been linked to seizures.
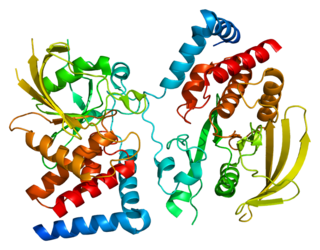
Receptor-type tyrosine-protein phosphatase gamma is an enzyme that in humans is encoded by the PTPRG gene.

Carbonic anhydrase-related protein 10 is an enzyme that in humans is encoded by the CA10 gene.

Carbonic anhydrase-related protein is a protein that in humans is encoded by the CA8 gene. The CA8 protein lacks the catalytic activity of other carbonic anhydrase enzymes. A rare, autosomal recessive form of cerebellar ataxia known as "cerebellar ataxia, mental retardation, and dysequilibrium syndrome 3" (CAMRQ3) is caused by mutations in the CA8 gene.
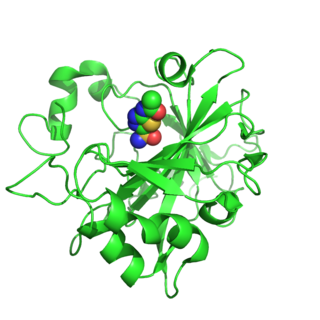
Carbonic anhydrase 14 is an enzyme that in humans is encoded by the CA14 gene.

Carbonic anhydrase 5B, mitochondrial is an enzyme that in humans is encoded by the CA5B gene.

Carbonic anhydrase-related protein 11 is a protein that in humans is encoded by the CA11 gene.

William S. Sly is an internationally known physician and scientist who, except for sabbatical years at Oxford and Stanford, spent his entire academic career in St. Louis. Following M.D. training at Saint Louis University School of Medicine, he trained in internal medicine at Washington University in St. Louis and in research laboratories at the NIH, in Paris, and in Madison, Wisconsin. He then joined the faculty at Washington University, where he directed the division of medical genetics for 20 years. In 1984, he was recruited to St. Louis University School of Medicine and appointed Alice A. Doisy Professor and chairman of the Edward A. Doisy Department of Biochemistry and Molecular Biology. He chaired that department for 26 years. In February 2007, he was also named the inaugural holder of the James B. and Joan C. Peters Endowed Chair in Biochemistry and Molecular Biology. He became an emeritus professor in July 2014.

Carbonic anhydrase 13 is a protein that in humans is encoded by the CA13 gene.

Carbonic anhydrase 5A, mitochondrial is a protein that in humans is encoded by the CA5A gene.