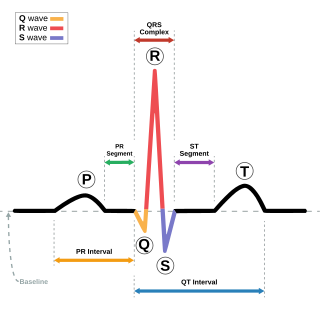
Electrocardiography is the process of producing an electrocardiogram. It is a graph of voltage versus time of the electrical activity of the heart using electrodes placed on the skin. These electrodes detect the small electrical changes that are a consequence of cardiac muscle depolarization followed by repolarization during each cardiac cycle (heartbeat). Changes in the normal ECG pattern occur in numerous cardiac abnormalities, including cardiac rhythm disturbances, inadequate coronary artery blood flow, and electrolyte disturbances.

Tachycardia, also called tachyarrhythmia, is a heart rate that exceeds the normal resting rate. In general, a resting heart rate over 100 beats per minute is accepted as tachycardia in adults. Heart rates above the resting rate may be normal or abnormal.

Long QT syndrome (LQTS) is a condition in which repolarization of the heart after a heartbeat is affected. It results in an increased risk of an irregular heartbeat which can result in fainting, drowning, seizures, or sudden death. These episodes can be triggered by exercise or stress. Some rare forms of LQTS are associated with other symptoms and signs including deafness and periods of muscle weakness.

The term micro-g environment is more or less synonymous with the terms weightlessness and zero-g, but with an emphasis on the fact that g-forces are never exactly zero—just very small. The symbol for microgravity, μg, was used on the insignias of Space Shuttle flights STS-87 and STS-107, because these flights were devoted to microgravity research in low Earth orbit.

Antiarrhythmic agents, also known as cardiac dysrhythmia medications, are a group of pharmaceuticals that are used to suppress abnormal rhythms of the heart, such as atrial fibrillation, atrial flutter, ventricular tachycardia, and ventricular fibrillation.

Short QT syndrome (SQT) is a very rare genetic disease of the electrical system of the heart, and is associated with an increased risk of abnormal heart rhythms and sudden cardiac death. The syndrome gets its name from a characteristic feature seen on an electrocardiogram (ECG) – a shortening of the QT interval. It is caused by mutations in genes encoding ion channels that shorten the cardiac action potential, and appears to be inherited in an autosomal dominant pattern. The condition is diagnosed using a 12-lead ECG. Short QT syndrome can be treated using an implantable cardioverter-defibrillator or medications including quinidine. Short QT syndrome was first described in 2000, and the first genetic mutation associated with the condition was identified in 2004.
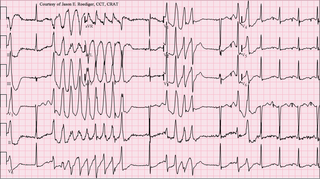
Torsades de pointes, torsade de pointes or torsades des pointes (TdP) is a specific type of abnormal heart rhythm that can lead to sudden cardiac death. It is a polymorphic ventricular tachycardia that exhibits distinct characteristics on the electrocardiogram (ECG). It was described by French physician François Dessertenne in 1966. Prolongation of the QT interval can increase a person's risk of developing this abnormal heart rhythm, occurring in between 1% and 10% of patients who receive QT-prolonging antiarrhythmic drugs.

Ventricular tachycardia is a type of regular, fast heart rate that arises from improper electrical activity in the ventricles of the heart. Although a few seconds may not result in problems, longer periods are dangerous; and multiple episodes over a short period of time is referred to as an Electrical Storm. Short periods may occur without symptoms, or present with lightheadedness, palpitations, or chest pain. Ventricular tachycardia may result in ventricular fibrillation and turn into cardiac arrest. It is found initially in about 7% of people in cardiac arrest.
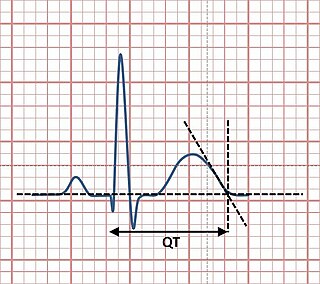
The QT interval is a measurement made on an electrocardiogram used to assess some of the electrical properties of the heart. It is calculated as the time from the start of the Q wave to the end of the T wave, and approximates to the time taken from when the cardiac ventricles start to contract to when they finish relaxing. An abnormally long or abnormally short QT interval is associated with an increased risk of developing abnormal heart rhythms and sudden cardiac death. Abnormalities in the QT interval can be caused by genetic conditions such as long QT syndrome, by certain medications such as sotalol or pitolisant, by disturbances in the concentrations of certain salts within the blood such as hypokalaemia, or by hormonal imbalances such as hypothyroidism.

Sotalol, sold under the brand name Betapace among others, is a medication used to treat and prevent abnormal heart rhythms. It is only recommended in those with significant abnormal heart rhythms due to potentially serious side effects. Evidence does not support a decreased risk of death with long term use. It is taken by mouth or injection into a vein.
Jervell and Lange-Nielsen syndrome (JLNS) is a rare type of long QT syndrome associated with severe, bilateral sensorineural hearing loss. Those with JLNS are at risk of abnormal heart rhythms called arrhythmias, which can lead to fainting, seizures, or sudden death. JLNS, like other forms of long QT syndrome, causes the cardiac muscle to take longer than usual to recharge between beats. It is caused by genetic variants responsible for producing ion channels that carry transport potassium out of cells. The condition is usually diagnosed using an electrocardiogram, but genetic testing can also be used. Treatment includes lifestyle measures, beta blockers, and implantation of a defibrillator in some cases. It was first described by Anton Jervell and Fred Lange-Nielsen in 1957.

Romano–Ward syndrome is the most common form of congenital Long QT syndrome (LQTS), a genetic heart condition that affects the electrical properties of heart muscle cells. Those affected are at risk of abnormal heart rhythms which can lead to fainting, seizures, or sudden death. Romano–Ward syndrome can be distinguished clinically from other forms of inherited LQTS as it affects only the electrical properties of the heart, while other forms of LQTS can also affect other parts of the body.

Andersen–Tawil syndrome, also called Andersen syndrome and long QT syndrome 7, is a rare genetic disorder affecting several parts of the body. The three predominant features of Andersen–Tawil syndrome include disturbances of the electrical function of the heart characterised by an abnormality seen on an electrocardiogram and a tendency to abnormal heart rhythms, physical characteristics including low-set ears and a small lower jaw, and intermittent periods of muscle weakness known as hypokalaemic periodic paralysis.
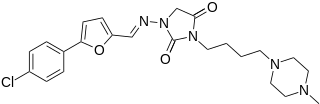
Azimilide is a class ΙΙΙ antiarrhythmic drug. The agents from this heterogeneous group have an effect on the repolarization, they prolong the duration of the action potential and the refractory period. Also they slow down the spontaneous discharge frequency of automatic pacemakers by depressing the slope of diastolic depolarization. They shift the threshold towards zero or hyperpolarize the membrane potential. Although each agent has its own properties and will have thus a different function.

Lorcainide is a Class 1c antiarrhythmic agent that is used to help restore normal heart rhythm and conduction in patients with premature ventricular contractions, ventricular tachycardiac and Wolff-Parkinson-White syndrome. Lorcainide was developed by Janssen Pharmaceutica (Belgium) in 1968 under the commercial name Remivox and is designated by code numbers R-15889 or Ro 13-1042/001. It has a half-life of 8.9 +- 2.3 hrs which may be prolonged to 66 hrs in people with cardiac disease.

Arrhythmia, also known as cardiac arrhythmia or heart arrhythmia, is a group of conditions in which the heartbeat is irregular, too fast, or too slow. The heart rate that is too fast – above 100 beats per minute in adults – is called tachycardia, and a heart rate that is too slow – below 60 beats per minute – is called bradycardia. Some types of arrhythmias have no symptoms. Symptoms, when present, may include palpitations or feeling a pause between heartbeats. In more serious cases, there may be lightheadedness, passing out, shortness of breath or chest pain. While most types of arrhythmia are not serious, some predispose a person to complications such as stroke or heart failure. Others may result in sudden death.

Celivarone is an experimental drug being tested for use in pharmacological antiarrhythmic therapy. Cardiac arrhythmia is any abnormality in the electrical activity of the heart. Arrhythmias range from mild to severe, sometimes causing symptoms like palpitations, dizziness, fainting, and even death. They can manifest as slow (bradycardia) or fast (tachycardia) heart rate, and may have a regular or irregular rhythm.
Illnesses and injuries during space missions are a range of medical conditions and injuries that may occur during space flights. Some of these medical conditions occur due to the changes withstood by the human body during space flight itself, while others are injuries that could have occurred on Earth's surface. A non-exhaustive list of these conditions and their probability of occurrence can be found in the following sources:
Skeletal muscles, particularly postural muscles of the lower limb, undergo atrophy and structural and metabolic alterations during space flight. The relationships between in-flight exercise, muscle changes and performance are not well understood. Efforts should be made to try to understand the current status of in-flight and post-flight exercise performance capacity and what the goals/target areas for protection are with the current in flight exercise program.
QT prolongation is a measure of delayed ventricular repolarisation, which means the heart muscle takes longer than normal to recharge between beats. It is an electrical disturbance which can be seen on an electrocardiogram (ECG). Excessive QT prolongation can trigger tachycardias such as torsades de pointes (TdP). QT prolongation is an established side effect of anti-arrhythmic medicines, but can also be caused by a wide range of non-cardiac medicines, including antibiotics, antihistamines, opioid analgesics and complementary medicines. On an EKG, the QT interval represents the summation of action potentials in cardiac muscle cells, which can be caused by an increase in inward current through sodium or calcium channels, or a decrease in outward current through potassium channels. By binding to and inhibiting the “rapid” delayed rectifier potassium current protein, certain drugs are able to decrease the outward flow of potassium ions and extend the length of phase 3 myocardial repolarization, resulting in QT prolongation.



![Figure 4. Left ventricular mass before and after short-duration space flight [based on(8), n=38]. * = P <= 0.05. Cardiac Figure 4.jpg](http://upload.wikimedia.org/wikipedia/commons/b/b6/Cardiac_Figure_4.jpg)














