
The Marchantiophyta are a division of non-vascular land plants commonly referred to as hepatics or liverworts. Like mosses and hornworts, they have a gametophyte-dominant life cycle, in which cells of the plant carry only a single set of genetic information.

Hornworts are a group of non-vascular Embryophytes constituting the division Anthocerotophyta. The common name refers to the elongated horn-like structure, which is the sporophyte. As in mosses and liverworts, hornworts have a gametophyte-dominant life cycle, in which cells of the plant carry only a single set of genetic information; the flattened, green plant body of a hornwort is the gametophyte stage of the plant.

Marchantiales is an order of thallose liverworts that includes species like Marchantia polymorpha, a widespread plant often found beside rivers, and Lunularia cruciata, a common and often troublesome weed in moist, temperate gardens and greenhouses.

Lunularia cruciata, the crescent-cup liverwort, is a liverwort of the order Marchantiales, and the only species in the genus Lunularia and family Lunulariaceae. The name, from Latin luna, moon, refers to the moon-shaped gemma cups.

Metzgeriales is an order of liverworts. The group is sometimes called the simple thalloid liverworts: "thalloid" because the members lack structures resembling stems or leaves, and "simple" because their tissues are thin and relatively undifferentiated. All species in the order have a small gametophyte stage and a smaller, relatively short-lived, spore-bearing stage. Although these plants are almost entirely restricted to regions with high humidity or readily available moisture, the group as a whole is widely distributed, and occurs on every continent except Antarctica.

Takakia is a genus of two species of mosses known from western North America and central and eastern Asia. The genus is placed as a separate family, order and class among the mosses. It has had a history of uncertain placement, but the discovery of sporophytes clearly of the moss-type firmly supports placement with the mosses.

Dendroceros is a genus of hornworts in the family Dendrocerotaceae. The genus contains about 51 species native to tropical and sub-tropical regions of the world.

Jungermanniopsida is the largest of three classes within the division Marchantiophyta (liverworts).
Monoicy is a sexual system in haploid plants where both sperm and eggs are produced on the same gametophyte, in contrast with dioicy, where each gametophyte produces only sperm or eggs but never both. Both monoicous and dioicous gametophytes produce gametes in gametangia by mitosis rather than meiosis, so that sperm and eggs are genetically identical with their parent gametophyte.

Buxbaumia is a genus of twelve species of moss (Bryophyta). It was first named in 1742 by Albrecht von Haller and later brought into modern botanical nomenclature in 1801 by Johann Hedwig to commemorate Johann Christian Buxbaum, a German physician and botanist who discovered the moss in 1712 at the mouth of the Volga River. The moss is microscopic for most of its existence, and plants are noticeable only after they begin to produce their reproductive structures. The asymmetrical spore capsule has a distinctive shape and structure, some features of which appear to be transitional from those in primitive mosses to most modern mosses.

Treubiaceae is a family of liverworts in the order Treubiales. Species are large and leafy, and were previously classified among the Metzgeriales.
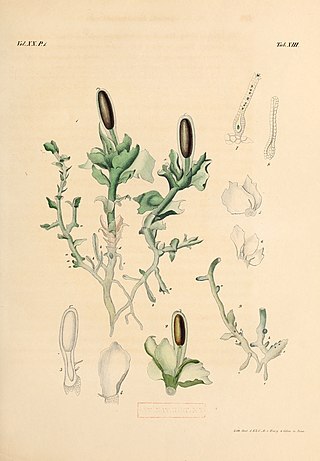
Haplomitriopsida is a newly recognized class of liverworts comprising fifteen species in three genera. Recent cladistic analyses of nuclear, mitochondrial, and plastid gene sequences place this monophyletic group as the basal sister group to all other liverworts. The group thus provides a unique insight into the early evolution of liverworts in particular and of land plants in general.

Blasiales is an order of liverworts with a single living family and two species. The order has traditionally been classified among the Metzgeriales, but molecular cladistics suggests a placement at the base of the Marchantiopsida.

Treubia is a genus of liverworts in the family Treubiaceae. There are seven species, all of which are restricted to the southern hemisphere. Five of the species occur in Australasia and the other occurs in Chile. All species are dioicous, with separate male and female gametophytes.

Ptilidium is a genus of liverwort, and is the only genus in family Ptilidiaceae. It includes only three species: Ptilidium californicum, Ptilidium ciliare, and Ptilidium pulcherrimum. The genus is distributed throughout the arctic and subarctic, with disjunct populations in New Zealand and Tierra del Fuego. Molecular analysis suggests that the genus has few close relatives and diverged from other leafy liverworts early in their evolution.
Neotrichocoleaceae is a family of liverworts in order Ptilidiales. It is closely related to the genera Ptilidium and Herzogianthus.

Riella is a genus in the liverwort family Riellaceae, and includes about eighteen species. Plants in the genus are small and grow submerged in shallow temporary pools. Although the genus is widely distributed in the Northern Hemisphere, locating populations is often difficult. Its occurrence is sporadic and local, and the tiny plants are ephemeral. The ornamented spores remain viable for several years, allowing the plants to survive annual drying of their habitat. The plants are easily grown in laboratory cultures.
Petalophyllum, or petalwort, is a genus of liverworts in the order Fossombroniales.
Dioicy is a sexual system where archegonia and antheridia are produced on separate gametophytes. It is one of the two main sexual systems in bryophytes, the other being monoicy. Both dioicous and monoicous gametophytes produce gametes in gametangia by mitosis rather than meiosis, so that sperm and eggs are genetically identical with their parent gametophyte.

Monoclea forsteri is one of the two species in the thallose liverwort family Monocleaceae. It is dioicous with the capsule dehiscing with a single longitudinal slit. Endemic and widely distributed throughout New Zealand, it is also the country's largest thalloid liverwort. Hooker described the species in 1820. The holotype is in the British Museum.















