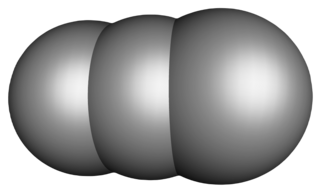Propane is a three-carbon alkane with the molecular formula C3H8. It is a gas at standard temperature and pressure, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as a fuel in domestic and industrial applications and in low-emissions public transportation. Discovered in 1857 by the French chemist Marcellin Berthelot, it became commercially available in the US by 1911. Propane is one of a group of liquefied petroleum gases. The others include butane, propylene, butadiene, butylene, isobutylene, and mixtures thereof. Propane has lower volumetric energy density, but higher gravimetric energy density and burns more cleanly than gasoline and coal.

Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and propane.

Halothane, sold under the brand name Fluothane among others, is a general anaesthetic. It can be used to induce or maintain anaesthesia. One of its benefits is that it does not increase the production of saliva, which can be particularly useful in those who are difficult to intubate. It is given by inhalation.
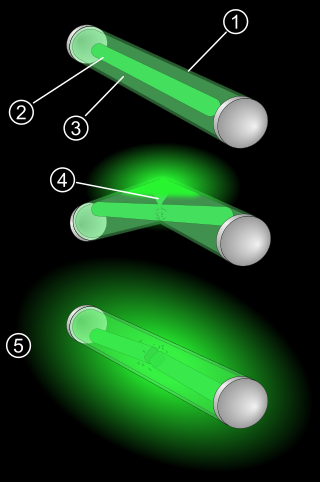
A glow stick, also known as a light stick, chem light, light wand, light rod, and rave light, is a self-contained, short-term light-source. It consists of a translucent plastic tube containing isolated substances that, when combined, make light through chemiluminescence. The light cannot be turned off and can be used only once. The used tube is then thrown away. Glow sticks are often used for recreation, such as for events, camping, outdoor exploration, and concerts. Glow sticks are also used for light in military and emergency services applications. Industrial uses include marine, transportation, and mining.

The organic compound 1,1,1-trichloroethane, also known as methyl chloroform and chlorothene, is a chloroalkane with the chemical formula CH3CCl3. It is an isomer of 1,1,2-trichloroethane. This colorless, sweet-smelling liquid was once produced industrially in large quantities for use as a solvent. It is regulated by the Montreal Protocol as an ozone-depleting substance and its use is being rapidly phased out.

Trichloroethylene (TCE) is a halocarbon with the formula C2HCl3, commonly used as an industrial degreasing solvent. It is a clear, colourless non-flammable liquid with a chloroform-like sweet smell.

Liquefied petroleum gas is a fuel gas which contains a flammable mixture of hydrocarbon gases, specifically propane, propylene, butylene, isobutane, and n-butane.

A boiling liquid expanding vapor explosion is an explosion caused by the rupture of a vessel containing a pressurized liquid that has reached a temperature above its boiling point. Because the boiling point of a liquid rises with pressure, the contents of the pressurized vessel can remain a liquid as long as the vessel is intact. If the vessel's integrity is compromised, the loss of pressure drops the boiling point, which can cause the liquid to convert to a gas expanding rapidly. If the gas is combustible, as in the case with hydrocarbons and alcohols, further damage can be caused by the ensuing fire.
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula CH3CH=CH2. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petroleum-like odor.

MAPP gas was a trademarked name, belonging to The Linde Group, a division of the former global chemical giant Union Carbide, for a fuel gas based on a stabilized mixture of methylacetylene (propyne), propadiene and propane. The name comes from the original chemical composition, methylacetylene-propadiene propane. "MAPP gas" is also widely used as a generic name for UN 1060 stabilised methylacetylene-propadiene.
The chemical compound 1,2-dichloroethane, commonly known as ethylene dichloride (EDC), is a chlorinated hydrocarbon. It is a colourless liquid with a chloroform-like odour. The most common use of 1,2-dichloroethane is in the production of vinyl chloride, which is used to make polyvinyl chloride (PVC) pipes, furniture and automobile upholstery, wall coverings, housewares, and automobile parts. 1,2-Dichloroethane is also used generally as an intermediate for other organic chemical compounds, and as a solvent. It forms azeotropes with many other solvents, including water and other chlorocarbons.
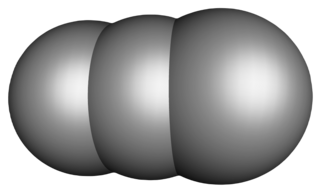
Tricarbon is an inorganic compound with the chemical formula C
2(μ-C). It is a colourless gas that only persists in dilution or solution as an adduct. It is one of the simplest unsaturated carbenes. Tricarbon can be found in interstellar space and can be produced in the laboratory by a process called laser ablation.
1,1-Dichloroethene, commonly called 1,1-dichloroethylene or vinylidene chloride or 1,1-DCE, is an organochloride with the molecular formula C2H2Cl2. It is a colorless liquid with a sharp odor. Like most chlorocarbons, it is poorly soluble in water, but soluble in organic solvents. 1,1-DCE was the precursor to the original clingwrap, Saran, for food, but this application has been phased out.

Methoxychlor is a synthetic organochloride insecticide, now obsolete. Tradenames for methoxychlor include Chemform, Maralate, Methoxo, Methoxcide, Metox, and Moxie.

Butane or n-butane is an alkane with the formula C4H10. Butane is a highly flammable, colorless, easily liquefied gas that quickly vaporizes at room temperature and pressure. The name butane comes from the root but- (from butyric acid, named after the Greek word for butter) and the suffix -ane. It was discovered in crude petroleum in 1864 by Edmund Ronalds, who was the first to describe its properties, and commercialized by Walter O. Snelling in early 1910s.
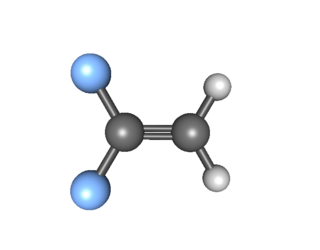
1,1-Difluoroethylene, also known as vinylidene fluoride, is a hydrofluoroolefin. It is a flammable gas. Global production in 1999 was approximately 33,000 metric tons. It is primarily used in the production of fluoropolymers such as polyvinylidene fluoride.
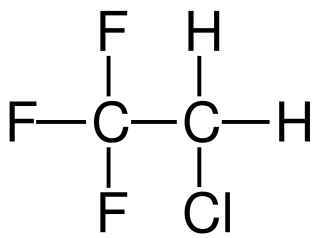
2-Chloro-1,1,1-trifluoroethane, also known as 1,1,1-trifluoro-2-chloroethane or Freon 133a, is an alkyl halide belonging to the category of chlorofluorocarbons, having chemical formula F3C-CH2-Cl. Under standard conditions, it appears as a colorless gas, partially soluble in water. It is used as a refrigerant, as a solvent and as a reagent in organic synthesis.
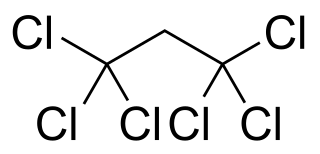
1,1,1,3,3,3-Hexachloropropane is a compound of chlorine, hydrogen, and carbon, with chemical formula C3Cl6H2, specifically Cl3C−CH2−CCl3. Its molecule can be described as that of propane with chlorine atoms substituted for the six hydrogen atoms on the extremal carbons.