Sodium laureth sulfate (SLES), an accepted contraction of sodium lauryl ether sulfate (SLES), also called sodium alkylethersulfate, is an anionic detergent and surfactant found in many personal care products and for industrial uses. SLES is an inexpensive and very effective foaming agent. SLES, sodium lauryl sulfate (SLS), ammonium lauryl sulfate (ALS), and sodium pareth sulfate are surfactants that are used in many cosmetic products for their cleaning and emulsifying properties. It is derived from palm kernel oil or coconut oil. In herbicides, it is used as a surfactant to improve absorption of the herbicidal chemicals and reduces time the product takes to be rainfast, when enough of the herbicidal agent will be absorbed.

Titration is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte. A reagent, termed the titrant or titrator, is prepared as a standard solution of known concentration and volume. The titrant reacts with a solution of analyte to determine the analyte's concentration. The volume of titrant that reacted with the analyte is termed the titration volume.

An alum is a type of chemical compound, usually a hydrated double sulfate salt of aluminium with the general formula XAl(SO
4)
2·12 H
2O, where X is a monovalent cation such as potassium or ammonium. By itself, "alum" often refers to potassium alum, with the formula KAl(SO
4)
2·12 H
2O. Other alums are named after the monovalent ion, such as sodium alum and ammonium alum.
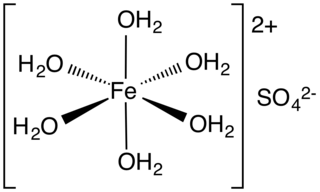
Iron(II) sulfate (British English: iron(II) sulphate) or ferrous sulfate denotes a range of salts with the formula Fe SO4·xH2O. These compounds exist most commonly as the heptahydrate (x = 7) but several values for x are known. The hydrated form is used medically to treat iron deficiency, and also for industrial applications. Known since ancient times as copperas and as green vitriol (vitriol is an archaic name for sulfate), the blue-green heptahydrate (hydrate with 7 molecules of water) is the most common form of this material. All the iron(II) sulfates dissolve in water to give the same aquo complex [Fe(H2O)6]2+, which has octahedral molecular geometry and is paramagnetic. The name copperas dates from times when the copper(II) sulfate was known as blue copperas, and perhaps in analogy, iron(II) and zinc sulfate were known respectively as green and white copperas.

An oxidizing agent is a substance in a redox chemical reaction that gains or "accepts"/"receives" an electron from a reducing agent. In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide and the halogens.

Photographic processing or photographic development is the chemical means by which photographic film or paper is treated after photographic exposure to produce a negative or positive image. Photographic processing transforms the latent image into a visible image, makes this permanent and renders it insensitive to light.

The E-6 process is a chromogenic photographic process for developing Ektachrome, Fujichrome and other color reversal photographic film.

Copper(II) sulfate, also known as copper sulphate, is an inorganic compound with the chemical formula CuSO4. It forms hydrates CuSO4·nH2O, where n can range from 1 to 7. The pentahydrate (n = 5), a bright blue crystal, is the most commonly encountered hydrate of copper(II) sulfate. Older names for the pentahydrate include blue vitriol, bluestone, vitriol of copper, and Roman vitriol. It exothermically dissolves in water to give the aquo complex [Cu(H2O)6]2+, which has octahedral molecular geometry. The structure of the solid pentahydrate reveals a polymeric structure wherein copper is again octahedral but bound to four water ligands. The Cu(II)(H2O)4 centers are interconnected by sulfate anions to form chains. Anhydrous copper sulfate is a light grey powder.

Calcium sulfate (or calcium sulphate) is the inorganic compound with the formula CaSO4 and related hydrates. In the form of γ-anhydrite (the anhydrous form), it is used as a desiccant. One particular hydrate is better known as plaster of Paris, and another occurs naturally as the mineral gypsum. It has many uses in industry. All forms are white solids that are poorly soluble in water. Calcium sulfate causes permanent hardness in water.

Barium sulfate (or sulphate) is the inorganic compound with the chemical formula BaSO4. It is a white crystalline solid that is odorless and insoluble in water. It occurs as the mineral barite, which is the main commercial source of barium and materials prepared from it. The white opaque appearance and its high density are exploited in its main applications.

Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula Na2SO4 as well as several related hydrates. All forms are white solids that are highly soluble in water. With an annual production of 6 million tonnes, the decahydrate is a major commodity chemical product. It is mainly used as a filler in the manufacture of powdered home laundry detergents and in the Kraft process of paper pulping for making highly alkaline sulfides.

Anhydrite, or anhydrous calcium sulfate, is a mineral with the chemical formula CaSO4. It is in the orthorhombic crystal system, with three directions of perfect cleavage parallel to the three planes of symmetry. It is not isomorphous with the orthorhombic barium (baryte) and strontium (celestine) sulfates, as might be expected from the chemical formulas. Distinctly developed crystals are somewhat rare, the mineral usually presenting the form of cleavage masses. The Mohs hardness is 3.5, and the specific gravity is 2.9. The color is white, sometimes greyish, bluish, or purple. On the best developed of the three cleavages, the lustre is pearly; on other surfaces it is glassy. When exposed to water, anhydrite readily transforms to the more commonly occurring gypsum, (CaSO4·2H2O) by the absorption of water. This transformation is reversible, with gypsum or calcium sulfate hemihydrate forming anhydrite by heating to around 200 °C (400 °F) under normal atmospheric conditions. Anhydrite is commonly associated with calcite, halite, and sulfides such as galena, chalcopyrite, molybdenite, and pyrite in vein deposits.

Potassium dichromate, K2Cr2O7, is a common inorganic chemical reagent, most commonly used as an oxidizing agent in various laboratory and industrial applications. As with all hexavalent chromium compounds, it is acutely and chronically harmful to health. It is a crystalline ionic solid with a very bright, red-orange color. The salt is popular in laboratories because it is not deliquescent, in contrast to the more industrially relevant salt sodium dichromate.

Aluminium sulfate is a salt with the formula Al2(SO4)3. It is soluble in water and is mainly used as a coagulating agent (promoting particle collision by neutralizing charge) in the purification of drinking water and wastewater treatment plants, and also in paper manufacturing.

Iron(III) sulfate (or ferric sulfate), is a family of inorganic compounds with the formula Fe2(SO4)3(H2O)n. A variety of hydrates are known, including the most commonly encountered form of "ferric sulfate". Solutions are used in dyeing as a mordant, and as a coagulant for industrial wastes. Solutions of ferric sulfate are also used in the processing of aluminum and steel.

Ammonium iron(III) sulfate, NH4Fe(SO4)2·12 H2O, or NH4[Fe(H2O)6](SO4)2·6 H2O, also known as ferric ammonium sulfate (FAS) or iron alum, is a double salt in the class of alums, which consists of compounds with the general formula AB(SO4)2 · 12 H2O. It has the appearance of weakly violet, octahedrical crystals. There has been some discussion regarding the origin of the crystals' colour, with some ascribing it to impurities in the compound, and others claiming it to be a property of the crystal itself.

The third in the series of color developing agents used in developing color films, commonly known as CD-3, is chemically known as N-[2-[(4-Amino-3-methylphenyl)ethylamino]ethyl]methanesulfonamide Sesquisulfate Monohydrate. In color development, after reducing a silver atom in a silver halide crystal, the oxidized developing agent combines with a color coupler to form a color dye molecule. CD-3 is used in many processes including VNF-1 and the E-6 process.

Color Developing Agent 2 is the second in the series of color developing agents used in developing color films. It is commonly known as CD-2 and is chemically known as 4-diethylamino-o-toluidine, 1,4-benzenediamine, N4,N4-diethyl-2-methyl-, N1,N1-diethyl-3-methylbenzene-1,4-diamine, or 4-(diethylamino)-2-methylaniline. In color development, after reducing a silver atom in a silver halide crystal, the oxidized developing agent combines with a color coupler to form a color dye molecule.

Color Developing Agent 1 (CD-1) is the first in the series of color developing agents used in developing color films. It is the organic compound N,N-diethyl-1,4-benzenediamine (DPD), which is usually in the form of the monohydrochloride salt. In color development, after reducing a silver atom in a silver halide crystal, the oxidized developing agent combines with a color coupler to form a color dye molecule.






















