
Ethers are a class of organic compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula R–O–R′, where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl or aryl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether" (CH3–CH2–O–CH2–CH3). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.
In organic chemistry, an alkyl substituent is an alkane missing one hydrogen. The term alkyl is intentionally unspecific to include many possible substitutions. An acyclic alkyl has the general formula of CnH2n+1. A cycloalkyl is derived from a cycloalkane by removal of a hydrogen atom from a ring and has the general formula CnH2n-1. Typically an alkyl is a part of a larger molecule. In structural formulae, the symbol R is used to designate a generic (unspecified) alkyl group. The smallest alkyl group is methyl, with the formula CH−3.
The E-6 process is a chromogenic photographic process for developing Ektachrome, Fujichrome and other color reversal photographic film.

The Williamson ether synthesis is an organic reaction, forming an ether from an organohalide and a deprotonated alcohol (alkoxide). This reaction was developed by Alexander Williamson in 1850. Typically it involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction. This reaction is important in the history of organic chemistry because it helped prove the structure of ethers.

Parathion, also called parathion-ethyl or diethyl parathion and locally known as "Folidol", is an organophosphate insecticide and acaricide. It was originally developed by IG Farben in the 1940s. It is highly toxic to non-target organisms, including humans, so its use has been banned or restricted in most countries. The basic structure is shared by parathion methyl.
The Michael reaction or Michael addition is the nucleophilic addition of a carbanion or another nucleophile to an α,β-unsaturated carbonyl compound containing an electron withdrawing group. It belongs to the larger class of conjugate additions and is widely used for the mild formation of C–C bonds. Many asymmetric variants exist.
The Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl functional group by formaldehyde and a primary or secondary amine or ammonia. The final product is a β-amino-carbonyl compound also known as a Mannich base. Reactions between aldimines and α-methylene carbonyls are also considered Mannich reactions because these imines form between amines and aldehydes. The reaction is named after Carl Mannich.
The agents in this list have been classified in group 2A by the International Agency for Research on Cancer (IARC). The term "agent" encompasses both substances and exposure circumstances that pose a risk. This designation is applied when there is limited evidence of carcinogenicity in humans as well as sufficient evidence of carcinogenicity in experimental animals. In some cases, an agent may be classified in this group when there is inadequate evidence of carcinogenicity in humans along with sufficient evidence of carcinogenicity in experimental animals and strong evidence that the carcinogenesis is mediated by a mechanism that also operates in humans. Exceptionally, an agent may be classified in this group solely on the basis of limited evidence of carcinogenicity in humans.

Diethyl azodicarboxylate, conventionally abbreviated as DEAD and sometimes as DEADCAT, is an organic compound with the structural formula CH3CH2O2CN=NCO2CH2CH3. Its molecular structure consists of a central azo functional group, RN=NR, flanked by two ethyl ester groups. This orange-red liquid is a valuable reagent but also quite dangerous and explodes upon heating. Therefore, commercial shipment of pure diethyl azodicarboxylate is prohibited in the United States and is carried out either in solution or on polystyrene particles.

The Controlled Drugs and Substances Act is Canada's federal drug control statute. Passed in 1996 under Prime Minister Jean Chrétien's government, it repeals the Narcotic Control Act and Parts III and IV of the Food and Drugs Act, and establishes eight Schedules of controlled substances and two Classes of precursors. It provides that "The Governor in Council may, by order, amend any of Schedules I to VIII by adding to them or deleting from them any item or portion of an item, where the Governor in Council deems the amendment to be necessary in the public interest."

A Grignard reagent or Grignard compound is a chemical compound with the generic formula R−Mg−X, where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride Cl−Mg−CH3 and phenylmagnesium bromide (C6H5)−Mg−Br. They are a subclass of the organomagnesium compounds.

Methyl vinyl ketone (MVK, IUPAC name: butenone) is the organic compound with the formula CH3C(O)CH=CH2. It is a reactive compound classified as an enone, in fact the simplest example thereof. It is a colorless, flammable, highly toxic liquid with a pungent odor. It is soluble in water and polar organic solvents. It is a useful intermediate in the synthesis of other compounds.
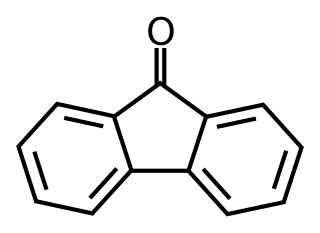
Fluorenone is an aromatic organic compound with the chemical formula C13H8O. It is used to make antimalaria drugs. It can be synthesised from fluorene with the addition of glacial acetic acid and sodium hypochlorite solution, undergoing an oxidation reaction. An alternate synthesis is described in scheme 43 of the Linopirdine patent.

Centralite (empirical formula: C17H20N2O) is a gunshot residue also known as ethyl centralite. Its IUPAC name is 1,3-diethyl-1,3-diphenylurea. Ethyl centralite is insoluble in water, but is soluble in acetone, ethanol and benzene. It is mainly used as a burning rate moderator and stabilizer for smokeless powder, and also a plasticizer for celluloid.

Pirimiphos-methyl, marketed as Actellic, and Sybol is a phosphorothioate used as an insecticide. It was originally developed by Imperial Chemical Industries Ltd., now Syngenta, at their Jealott's Hill site and first marketed in 1977, ten years after its discovery.

Isopropyl aminoethylmethyl phosphonite, also known as O-(2-diisopropylaminoethyl) O′-ethyl methylphosphonite, is a precursor chemical to the nerve agent VX and VR-56. It is a colorless liquid with a strong fishy odor, and is slightly soluble in water.

Color Developing Agent 1 (CD-1) is the first in the series of color developing agents used in developing color films. It is the organic compound N,N-diethyl-1,4-benzenediamine (DPD), which is usually in the form of the monohydrochloride salt. In color development, after reducing a silver atom in a silver halide crystal, the oxidized developing agent combines with a color coupler to form a color dye molecule.

Prenderol (Diethylpropanediol) is a simple alkyl diol which has sedative, anticonvulsant and muscle relaxant effects. It is closely related in structure to meprobamate and numerous other alkyl alcohols and diols with generally comparable activity.















