
Pseudoephedrine, sold under the brand name Sudafed among others, is a sympathomimetic medication which is used as a decongestant to treat nasal congestion. It has also been used off-label for certain other indications, like treatment of low blood pressure. At higher doses, it may produce various additional effects, including psychostimulant, appetite suppressant, and performance-enhancing effects. In relation to this, non-medical use of pseudoephedrine has been encountered. The medication is taken by mouth.

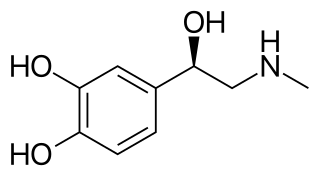
Ephedrine is a central nervous system (CNS) stimulant and sympathomimetic agent that is often used to prevent low blood pressure during anesthesia. It has also been used for asthma, narcolepsy, and obesity but is not the preferred treatment. It is of unclear benefit in nasal congestion. It can be taken by mouth or by injection into a muscle, vein, or just under the skin. Onset with intravenous use is fast, while injection into a muscle can take 20 minutes, and by mouth can take an hour for effect. When given by injection, it lasts about an hour, and when taken by mouth, it can last up to four hours.

Over-the-counter (OTC) drugs are medicines sold directly to a consumer without a requirement for a prescription from a healthcare professional, as opposed to prescription drugs, which may be supplied only to consumers possessing a valid prescription. In many countries, OTC drugs are selected by a regulatory agency to ensure that they contain ingredients that are safe and effective when used without a physician's care. OTC drugs are usually regulated according to their active pharmaceutical ingredient (API) and strengths of final products.

Loratadine, sold under the brand name Claritin among others, is a medication used to treat allergies. This includes allergic rhinitis and hives. It is also available in drug combinations such as loratadine/pseudoephedrine, in which it is combined with pseudoephedrine, a nasal decongestant. It is taken orally.

D-norpseudoephedrine, also known as cathine and (+)-norpseudoephedrine, is a psychoactive drug of the phenethylamine and amphetamine chemical classes which acts as a stimulant. Along with cathinone, it is found naturally in Catha edulis (khat), and contributes to its overall effects. It has approximately 7-10% the potency of amphetamine.

Clandestine chemistry is chemistry carried out in secret, and particularly in illegal drug laboratories. Larger labs are usually run by gangs or organized crime intending to produce for distribution on the black market. Smaller labs can be run by individual chemists working clandestinely in order to synthesize smaller amounts of controlled substances or simply out of a hobbyist interest in chemistry, often because of the difficulty in ascertaining the purity of other, illegally synthesized drugs obtained on the black market. The term clandestine lab is generally used in any situation involving the production of illicit compounds, regardless of whether the facilities being used qualify as a true laboratory.

Phenylpropanolamine (PPA), sold under many brand names, is a sympathomimetic agent which is used as a decongestant and appetite suppressant. It was previously commonly used in prescription and over-the-counter cough and cold preparations. The medication is taken by mouth.
Sympathomimetic drugs are stimulant compounds which mimic the effects of endogenous agonists of the sympathetic nervous system. Examples of sympathomimetic effects include increases in heart rate, force of cardiac contraction, and blood pressure. The primary endogenous agonists of the sympathetic nervous system are the catecholamines, which function as both neurotransmitters and hormones. Sympathomimetic drugs are used to treat cardiac arrest and low blood pressure, or even delay premature labor, among other things.
The ECA stack is a drug combination used in weight loss and as a stimulant. ECA is an initialism for ephedrine, caffeine, and aspirin, with variants of it including the EC stack, which removes the aspirin for those who can not tolerate it. Dietary supplements based on or including elements of ECA were popular through the 1990s and early 2000s, but the marketing of ephedra- or ephedrine-containing stimulant combinations for weight loss and bodybuilding is now restricted or illegal in the United States and the Netherlands due to reports of heart attack, stroke, and death associated with these supplements.
The Methamphetamine Precursor Control Act is an Illinois state law that was signed into law on November 16, 2005, and took effect on January 15, 2006. The MPCA is an act used to create significant barriers such as the requirement to present ID to purchase cold medication that contains pseudoephedrine which could be used to produce methamphetamine. This is one of many state laws regulating the supply of pseudoephedrine that preceded the federal Combat Methamphetamine Epidemic Act of 2005.

The Montana Meth Project (MMP) is a Montana-based non-profit organization founded by businessman Thomas Siebel which seeks to reduce methamphetamine use, particularly among teenagers. The organizations main approach includes television, radio, print, and internet public service announcements that graphically depict the negative consequences of methamphetamine use. Common elements are the deterioration of health and living conditions, amphetamine psychosis, moral compromise, and regret. As of 2010, the Meth Project has expanded its media campaign into seven additional states. In March 13, 2013, the Montana Meth Project, joined the Partnership for Drug-Free Kids.
Zhenli Ye Gon is a Chinese-Mexican businessman currently under suspicion of trafficking pseudoephedrine or ephedrine precursor chemicals into Mexico from Asia. He is the owner and legal representative of Unimed Pharm Chem México, as well as various other Mexican corporations. From 2002 to 2004, Unimed had been legally authorized by the Mexican government to import thousands of metric tons of pseudoephedrine and ephedrine products into Mexico, as a part of its vast importation business. Pseudoephedrine and ephedrine products at the time were widely used in over-the-counter cold medications such as Sudafed, but could also be used by manufacturers of methamphetamine.
The illegal drug trade in China is influenced by factors such as history, location, size, population, and current economic conditions. China has one-sixth of the world's population and a large and expanding economy. China's large land mass, close proximity to the Golden Triangle, Golden Crescent, and numerous coastal cities with large and modern port facilities make it an attractive transit center for drug traffickers. Opium has played an important role in the country's history since before the First and Second Opium Wars in the mid-19th century.
The U.S. state of Oregon has various policies restricting the production, sale, and use of different substances. In 2006, Oregon's per capita drug use exceeded the national average. The most used substances were marijuana, methamphetamine and illicit painkillers and stimulants.
The production, distribution, and sale of methamphetamine is restricted or illegal in many jurisdictions.

The Comprehensive Methamphetamine Control Act of 1996 is a bill enacted into law by the 104th Congress of the United States. It mandated registration of persons trading in list I chemicals from the DEA list of chemicals. A fee for such registration was initially $595 but later reduced to $116. It is regarded as one of the major drug laws in the United States.
Drug precursors, also referred to as precursor chemicals or simply precursors, are substances used to manufacture illicit drugs. Most precursors also have legitimate commercial uses and are legally used in a wide variety of industrial processes and consumer products, such as medicines, flavourings, and fragrances.

Methamphetamine in the United States is regulated under Schedule II of the Controlled Substances Act. It is approved for pharmacological use in the treatment of attention deficit hyperactivity disorder, narcolepsy, and treatment-resistant obesity, but it is primarily used as a recreational drug. In 2012, 16,000 prescriptions for methamphetamine were filled, approximately 1.2 million Americans reported using it in the past year, and 440,000 reported using the drug in the past month.
Amphetamine and methamphetamine are central nervous system stimulants used to treat a variety of conditions. When used recreationally, they are colloquially known as "speed" or sometimes "crank". Amphetamine was first synthesized in 1887 in Germany by Romanian chemist Lazăr Edeleanu, who named it phenylisopropylamine. Around the same time, Japanese organic chemist Nagai Nagayoshi isolated ephedrine from the Chinese ephedra plant and later developed a method for ephedrine synthesis. Methamphetamine was synthesized from ephedrine in 1893 by Nagayoshi. Neither drug had a pharmacological use until 1934, when Smith, Kline & French began selling amphetamine as an inhaler under the trade name Benzedrine for congestion.
The National Precursor Log Exchange (NPLEx) is a real-time electronic logging system which tracks sales of the methamphetamine precursors ephedrine and pseudoephedrine in the United States. The program is operated by the National Association of Drug Diversion Investigators (NADDI), funded by manufacturers of pseudoephedrine medicine, and technology provided through contract by Apriss Inc. As of January 2014, 29 states have passed NPLEx-related legislation, and it is implemented in most other states on a voluntary basis by national retail chains.











