Enriched uranium is a type of uranium in which the percent composition of uranium-235 has been increased through the process of isotope separation. Naturally occurring uranium is composed of three major isotopes: uranium-238, uranium-235, and uranium-234. 235U is the only nuclide existing in nature that is fissile with thermal neutrons.
A gas nuclear reactor is a proposed kind of nuclear reactor in which the nuclear fuel would be in a gaseous state rather than liquid or solid. In this type of reactor, the only temperature-limiting materials would be the reactor walls. Conventional reactors have stricter limitations because the core would melt if the fuel temperature were to rise too high. It may also be possible to confine gaseous fission fuel magnetically, electrostatically or electrodynamically so that it would not touch the reactor walls. A potential benefit of the gaseous reactor core concept is that instead of relying on the traditional Rankine or Brayton conversion cycles, it may be possible to extract electricity magnetohydrodynamically, or with simple direct electrostatic conversion of the charged particles.
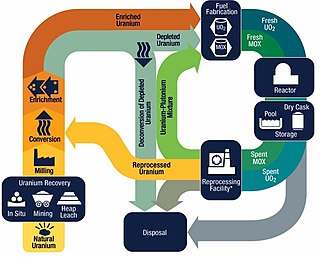
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the front end, which are the preparation of the fuel, steps in the service period in which the fuel is used during reactor operation, and steps in the back end, which are necessary to safely manage, contain, and either reprocess or dispose of spent nuclear fuel. If spent fuel is not reprocessed, the fuel cycle is referred to as an open fuel cycle ; if the spent fuel is reprocessed, it is referred to as a closed fuel cycle.
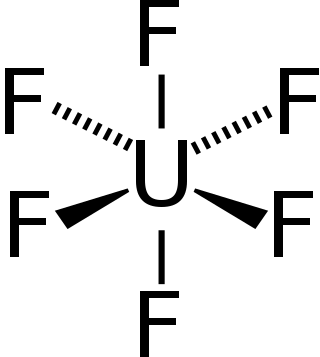
Uranium hexafluoride, sometimes called hex, is an inorganic compound with the formula UF6. Uranium hexafluoride is a volatile and toxic white solid that reacts with water, releasing corrosive hydrofluoric acid. The compound reacts mildly with aluminium, forming a thin surface layer of AlF3 that resists any further reaction from the compound. UF6 is used in the process of enriching uranium, which produces fuel for nuclear reactors and nuclear weapons.

Yellowcake is a type of powdered uranium concentrate obtained from leach solutions, in an intermediate step in the processing of uranium ores. It is a step in the processing of uranium after it has been mined but before fuel fabrication or uranium enrichment. Yellowcake concentrates are prepared by various extraction and refining methods, depending on the types of ores. Typically, yellowcakes are obtained through the milling and chemical processing of uranium ore, forming a coarse powder that has a pungent odor, is insoluble in water, and contains about 80% uranium oxide, which melts at approximately 2880 °C.

Gaseous diffusion is a technology that was used to produce enriched uranium by forcing gaseous uranium hexafluoride (UF6) through microporous membranes. This produces a slight separation (enrichment factor 1.0043) between the molecules containing uranium-235 (235U) and uranium-238 (238U). By use of a large cascade of many stages, high separations can be achieved. It was the first process to be developed that was capable of producing enriched uranium in industrially useful quantities, but is nowadays considered obsolete, having been superseded by the more-efficient gas centrifuge process (enrichment factor 1.05 to 1.2).

K-25 was the codename given by the Manhattan Project to the program to produce enriched uranium for atomic bombs using the gaseous diffusion method. Originally the codename for the product, over time it came to refer to the project, the production facility located at the Clinton Engineer Works in Oak Ridge, Tennessee, the main gaseous diffusion building, and ultimately the site. When it was built in 1944, the four-story K-25 gaseous diffusion plant was the world's largest building, comprising over 5,264,000 square feet (489,000 m2) of floor space and a volume of 97,500,000 cubic feet (2,760,000 m3).

The Honeywell Uranium Hexafluoride Processing Facility, a uranium conversion facility, is located 1.9 miles (3 km) northwest of Metropolis, Illinois, United States. The plant, Honeywell Specialty Chemicals in Metropolis, Illinois, has a nominal capacity of 15,000 tU as uranium hexafluoride per year. ConverDyn, a general partnership between affiliates of Honeywell and General Atomics, is the exclusive agent for conversion sales from the Honeywell Uranium Hexafluoride Processing Facility.
Fluoride volatility is the tendency of highly fluorinated molecules to vaporize at comparatively low temperatures. Heptafluorides, hexafluorides and pentafluorides have much lower boiling points than the lower-valence fluorides. Most difluorides and trifluorides have high boiling points, while most tetrafluorides and monofluorides fall in between. The term "fluoride volatility" is jargon used particularly in the context of separation of radionuclides.
Molecular laser isotope separation (MLIS) is a method of isotope separation, where specially tuned lasers are used to separate isotopes of uranium using selective ionization of hyperfine transitions of uranium hexafluoride molecules. It is similar to AVLIS. Its main advantage over AVLIS is low energy consumption and use of uranium hexafluoride instead of vaporized uranium. MLIS was conceived in 1971 at the Los Alamos National Laboratory.

Uranium tetrafluoride is the inorganic compound with the formula UF4. It is a green solid with an insignificant vapor pressure and low solubility in water. Uranium in its tetravalent (uranous) state is important in various technological processes. In the uranium refining industry it is known as green salt.
Separation of isotopes by laser excitation (SILEX) is a process for enriching uranium to fuel nuclear reactors that may also present a growing nuclear weapons proliferation risk. It is strongly suspected that SILEX utilizes laser condensation repression to excite a vibrational mode of the uranium-235 isotope in uranium hexaflouride (UF6), allowing this lighter molecule to move more rapidly to the outer rim of a gaseous jet and resist condensing compared to the heavier, unexcited 238UF6. This differs greatly from previous methods of laser enrichment explored for their commercial prospects: one using atomic uranium (Atomic Vapor Laser Isotope Separation (AVLIS)) and another molecular method that uses lasers to dissociate a fluorine atom from 235UF6 (Molecular Laser Isotope Separation (MLIS)), allowing the enriched product to precipitate out as a solid.
Uranium compounds are compounds formed by the element uranium (U). Although uranium is a radioactive actinide, its compounds are well studied due to its long half-life and its applications. It usually forms in the +4 and +6 oxidation states, although it can also form in other oxidation states.

The Fernald Feed Materials Production Center is a Superfund site located within Crosby Township in Hamilton County, Ohio, as well as Ross Township in Butler County, Ohio, in the United States. It was a uranium processing facility located near the rural town of New Baltimore, about 20 miles (32 km) northwest of Cincinnati, which fabricated uranium fuel cores for the U.S. nuclear weapons production complex from 1951 to 1989. During that time, the plant produced 170,000 metric tons uranium (MTU) of metal products and 35,000 MTU of intermediate compounds, such as uranium trioxide and uranium tetrafluoride.

Plutonium hexafluoride is the highest fluoride of plutonium, and is of interest for laser enrichment of plutonium, in particular for the production of pure plutonium-239 from irradiated uranium. This isotope of plutonium is needed to avoid premature ignition of low-mass nuclear weapon designs by neutrons produced by spontaneous fission of plutonium-240.
Sequoyah Fuels Corporation owned and operated a uranium processing plant near Gore, Oklahoma. The company was created in 1983 as a subsidiary of Kerr-McGee. In 1988 it was sold to General Atomics.

Neptunium(VI) fluoride (NpF6) is the highest fluoride of neptunium, it is also one of seventeen known binary hexafluorides. It is a volatile orange crystalline solid. It is relatively hard to handle, being very corrosive, volatile and radioactive. Neptunium hexafluoride is stable in dry air but reacts vigorously with water.

The Malvési uranium processing plant, a uranium refinery and conversion facility, is located in the Malvezy industrial area in the city of Narbonne in the south of France. The plant has an average capacity of about 14,000 tU as uranium tetrafluoride per year, and is projected to increase its capacity up to 21,000 tU per year. Comurhex, a subsidiary of the French nuclear concern Orano, operates the Malvési Facility.
Depleted uranium hexafluoride (DUHF; also referred to as depleted uranium tails, depleted uranium tailings or DUF6) is a byproduct of the processing of uranium hexafluoride into enriched uranium. It is one of the chemical forms of depleted uranium (up to 73-75%), along with depleted triuranium octoxide (up to 25%) and depleted uranium metal (up to 2%). DUHF is 1.7 times less radioactive than uranium hexafluoride and natural uranium.
The Uranium Conversion Facility in Islamabad is a uranium conversion site in Islamabad, Pakistan. The plant is owned by the Pakistan Atomic Energy Commission and has a nominal capacity of producing uranium hexafluoride (UF6).
















