
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a substructure of the organism, such as a cell (cytotoxicity) or an organ such as the liver (hepatotoxicity). By extension, the word may be metaphorically used to describe toxic effects on larger and more complex groups, such as the family unit or society at large. Sometimes the word is more or less synonymous with poisoning in everyday usage.
Cresols are a group of aromatic organic compounds. They are widely-occurring phenols which may be either natural or manufactured. They are also categorized as methyl phenols. Cresols commonly occur as either solids or liquids because their melting points are generally close to room temperature. Like other types of phenols, they are slowly oxidized by exposure to air, and the resulting impurities often give the samples a yellow to brownish red tint. Cresols have an odor characteristic to that of other simple phenols, reminiscent to some of a "coal tar" smell. The name "cresol" is an adduct of phenol and their traditional source, creosote.
The permissible exposure limit is a legal limit in the United States for exposure of an employee to a chemical substance or physical agent such as high level noise. Permissible exposure limits were established by the Occupational Safety and Health Administration (OSHA). Most of OSHA's PELs were issued shortly after adoption of the Occupational Safety and Health (OSH) Act in 1970.

Chemical hazards are typical of hazardous chemicals and hazardous materials in general. Exposure to certain chemicals can cause acute or long-term adverse health effects. Chemical hazards are usually classified separately from biological hazards (biohazards). Main classifications of chemical hazards include asphyxiants, corrosives, irritants, sensitizers, carcinogens, mutagens, teratogens, reactants, and flammables. In the workplace, exposure to chemical hazards is a type of occupational hazard. The use of protective personal equipment (PPE) may substantially reduce the risk of damage from contact with hazardous materials.
The threshold limit value (TLV) is believed to be a level to which a worker can be exposed per shift in the worktime without adverse effects. Strictly speaking, TLV is a reserved term of the American Conference of Governmental Industrial Hygienists (ACGIH). TLVs issued by the ACGIH are the most widely accepted occupational exposure limits both in the United States and most other countries. However, it is sometimes loosely used to refer to other similar concepts used in occupational health and toxicology, such as acceptable daily intake (ADI) and tolerable daily intake (TDI). Concepts such as TLV, ADI, and TDI can be compared to the no-observed-adverse-effect level (NOAEL) in animal testing, but whereas a NOAEL can be established experimentally during a short period, TLV, ADI, and TDI apply to human beings over a lifetime and thus are harder to test empirically and are usually set at lower levels. TLVs, along with biological exposure indices (BEIs), are published annually by the ACGIH.

p-Xylene (para-xylene) is an aromatic hydrocarbon. It is one of the three isomers of dimethylbenzene known collectively as xylenes. The p- stands for para-, indicating that the two methyl groups in p-xylene occupy the diametrically opposite substituent positions 1 and 4. It is in the positions of the two methyl groups, their arene substitution pattern, that it differs from the other isomers, o-xylene and m-xylene. All have the same chemical formula C6H4(CH3)2. All xylene isomers are colorless and highly flammable. The odor threshold of p-xylene is 0.62 parts per million (ppm).

Ethyl formate is an ester formed when ethanol reacts with formic acid. Ethyl formate has the characteristic smell of rum and is also partially responsible for the flavor of raspberries. It occurs naturally in the body of ants and in the stingers of bees.
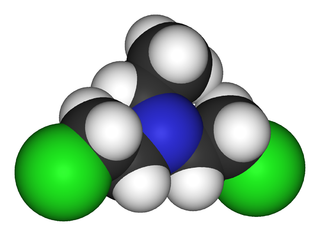
Bis(2-chloroethyl)ethylamine is the organic compound with the formula C2H5N(CH2CH2Cl)2. Often abbreviated HN1, it is a powerful vesicant and a nitrogen mustard gas used for chemical warfare. HN1 was developed in the 1920s and 1930s to remove warts and later as a military agent. Because of the latter use, it is a Schedule 1 chemical within the Chemical Weapons Convention and therefore use and production is strongly restricted. It has never been used in warfare.
Potassium nitrate is an oxidizer so storing it near fire hazards or reducing agents should be avoided to minimise risk in case of a fire.

Benzotrichloride (BTC), also known as α,α,α-trichlorotoluene, phenyl chloroform or (trichloromethyl)benzene, is an organic compound with the formula C6H5CCl3. Benzotrichloride is an unstable, colorless or somewhat yellowish, viscous, chlorinated hydrocarbon with a penetrating odor. Benzotrichloride is used extensively as a chemical intermediate for products of various classes, i.e. dyes and antimicrobial agents.
Control banding is a qualitative or semi-quantitative risk assessment and management approach to promoting occupational health and safety. It is intended to minimize worker exposures to hazardous chemicals and other risk factors in the workplace and to help small businesses by providing an easy-to-understand, practical approach to controlling hazardous exposures at work.

Manure management refers to capture, storage, treatment, and utilization of animal manures in an environmentally sustainable manner. It can be retained in various holding facilities. Animal manure can occur in a liquid, slurry, or solid form. It is utilized by distribution on fields in amounts that enrich soils without causing water pollution or unacceptably high levels of nutrient enrichment. Manure management is a component of nutrient management.
A short-term exposure limit (STEL) is the acceptable average exposure over a short period of time, usually 15 minutes as long as the time-weighted average is not exceeded.
Chlorine gas poisoning is an illness resulting from the effects of exposure to chlorine beyond the threshold limit value.

Tetramethyllead, also called tetra methyllead and lead tetramethyl, is a chemical compound used as an antiknock additive for gasoline. Its use is being phased out for environmental considerations.
The Zschiegner Refining Company (ZRC) was located in Howell, New Jersey, and operated as a metal refining facility. Some of their operations included stripping the chemicals off of precious metals from watch bands, photographic film, and electrical components. In 1992, the United States Environmental Protection Agency (EPA) discovered that 3,000 chemicals had contaminated the soil, surface water, and groundwater. These areas were contaminated due to discharge of waste to the ground surface, the movement of waste and contamination downhill from the site building, along with surface water runoff. When the EPA investigated the site and found the chemicals in the groundwater and soil, they shut down the company that same year in 1992. In that area, the environment and people were affected, especially the workers in the facility. After a Hazard Ranking System report was conducted by the EPA, the site was placed on the National Priorities List in March 1998. In 2008, the cleanup was completed, but groundwater and wetland is still being monitored.
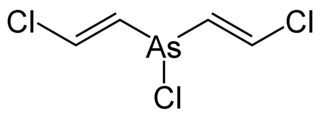
Lewisite 2(L-2) is an organoarsenic chemical weapon with the formula AsCl(CH=CHCl)2. It is similar to lewisite 1 and lewisite 3 and was first synthesized in 1904 by Julius Arthur Nieuwland. It is usually found as a mixture of 2-chlorovinylarsonous dichloride (lewisite 1) as well as bis(2-chloroethenyl) arsinous chloride (lewisite 2) and tris(2-chlorovinyl)arsine (lewisite 3). Pure lewisite 1 is an oily, colorless liquid, however, the impure mixture can appear amber to black with an odor distinct to geraniums.

Lewisite 3(L-3) is an organoarsenic chemical weapon like lewisite 1 and lewisite 2 first synthesized in 1904 by Julius Arthur Nieuwland. It is usually found as a mixture of 2-chlorovinylarsonous dichloride as well as bis(2-chloroethenyl) arsinous chloride and tris(2-chlorovinyl)arsine. Pure lewisite 1 is an oily, colorless liquid, however, the impure mixture can appear amber to black with an odor distinct to geraniums.
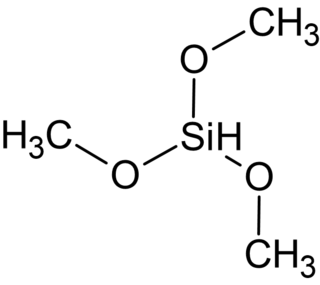
Trimethoxysilane (TMS) is an organosilicon compound with the formula HSi(OCH3)3. The compound is a commonly used basic raw material for the preparation of silicone materials.













