In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.

In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.
In organic chemistry, a nitrile is any organic compound that has a −C≡N functional group. The name of the compound is composed of a base, which includes the carbon of the −C≡N, suffixed with "nitrile", so for example CH3CH2C≡N is called "propionitrile". The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.

In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups. The nitro group is one of the most common explosophores used globally. The nitro group is also strongly electron-withdrawing. Because of this property, C−H bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature. They are almost invariably produced by nitration reactions starting with nitric acid.

Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2.

The Knorr pyrrole synthesis is a widely used chemical reaction that synthesizes substituted pyrroles (3). The method involves the reaction of an α-amino-ketone (1) and a compound containing an electron-withdrawing group α to a carbonyl group (2).

Cyanamide is an organic compound with the formula CN2H2. This white solid is widely used in agriculture and the production of pharmaceuticals and other organic compounds. It is also used as an alcohol-deterrent drug. The molecule features a nitrile group attached to an amino group. Derivatives of this compound are also referred to as cyanamides, the most common being calcium cyanamide (CaCN2).

Diphenylmethanol is the organic compound with the formula (C6H5)2CHOH. Also known as benzhydrol, it is a white solid and the parent member of a large class of diaryl alcohols.
Stephen aldehyde synthesis, a named reaction in chemistry, was invented by Henry Stephen (OBE/MBE). This reaction involves the preparation of aldehydes (R-CHO) from nitriles (R-CN) using tin(II) chloride (SnCl2), hydrochloric acid (HCl) and quenching the resulting iminium salt ([R-CH=NH2]+Cl−) with water (H2O). During the synthesis, ammonium chloride is also produced.

Ocinaplon is an anxiolytic drug in the pyrazolopyrimidine family of drugs. Other pyrazolopyrimidine drugs include zaleplon and indiplon.

Benzyl cyanide (abbreviated BnCN) is an organic compound with the chemical formula C6H5CH2CN. This colorless oily aromatic liquid is an important precursor to numerous compounds in organic chemistry. It is also an important pheromone in certain species.
In organic synthesis, cyanation is the attachment or substitution of a cyanide group on various substrates. Such transformations are high-value because they generate C-C bonds. Furthermore nitriles are versatile functional groups.
In nitrile reduction a nitrile is reduced to either an amine or an aldehyde with a suitable chemical reagent.
The Ritter reaction is a chemical reaction that transforms a nitrile into an N-alkyl amide using various electrophilic alkylating reagents. The original reaction formed the alkylating agent using an alkene in the presence of a strong acid.

Sodium chloroacetate is the organic compound with the formula CH2ClCO2Na. A white, water-soluble solid, it is the sodium salt of chloroacetic acid. Many of its uses are similar to those of the parent acid. It is prepared by treating chloroacetic acid with sodium carbonate.
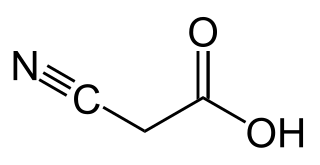
Cyanoacetic acid is an organic compound. It is a white, hygroscopic solid. The compound contains two functional groups, a nitrile (−C≡N) and a carboxylic acid. It is a precursor to cyanoacrylates, components of adhesives.

Ethyl cyanoacetate is an organic compound that contains a carboxylate ester and a nitrile. It is a colourless liquid with a pleasant odor. This material is useful as a starting material for synthesis due to its variety of functional groups and chemical reactivity.

Diethyl phosphite is the organophosphorus compound with the formula (C2H5O)2P(O)H. It is a popular reagent for generating other organophosphorus compounds, exploiting the high reactivity of the P-H bond. Diethyl phosphite is a colorless liquid. The molecule is tetrahedral.

Cyanoethylation is a process for the attachment of CH2CH2CN group to another organic substrate. The method is used in the synthesis of organic compounds.
4-Chlorobutyronitrile is the organic compound with the formula ClCH2CH2CH2CN. With both chloro and cyano functional groups, it is a bifunctional molecule. It is a colorless liquid.