
A polymer is a substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeating subunits derived from one or more species of monomers. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging (plastic bags, plastic films, geomembranes and containers including bottles, cups, jars, etc.). As of 2017, over 100 million tonnes of polyethylene resins are being produced annually, accounting for 34% of the total plastics market.

A thermoplastic, or thermosofteningplastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.

Polyethylene terephthalate (or poly(ethylene terephthalate), PET, PETE, or the obsolete PETP or PET-P), is the most common thermoplastic polymer resin of the polyester family and is used in fibres for clothing, containers for liquids and foods, and thermoforming for manufacturing, and in combination with glass fibre for engineering resins.
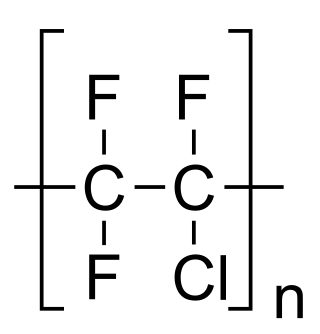
Polychlorotrifluoroethylene (PCTFE or PTFCE) is a thermoplastic chlorofluoropolymer with the molecular formula (CF2CClF)n, where n is the number of monomer units in the polymer molecule. It is similar to polytetrafluoroethene (PTFE), except that it is a homopolymer of the monomer chlorotrifluoroethylene (CTFE) instead of tetrafluoroethene. It has the lowest water vapor transmission rate of any plastic.

High-density polyethylene (HDPE) or polyethylene high-density (PEHD) is a thermoplastic polymer produced from the monomer ethylene. It is sometimes called "alkathene" or "polythene" when used for HDPE pipes. With a high strength-to-density ratio, HDPE is used in the production of plastic bottles, corrosion-resistant piping, geomembranes and plastic lumber. HDPE is commonly recycled, and has the number "2" as its resin identification code.

An ionomer is a polymer composed of repeat units of both electrically neutral repeating units and ionized units covalently bonded to the polymer backbone as pendant group moieties. Usually no more than 15 mole percent are ionized. The ionized units are often carboxylic acid groups.
A polyolefin is a type of polymer with the general formula (CH2CHR)n where R is an alkyl group. They are usually derived from a small set of simple olefins (alkenes). Dominant in a commercial sense are polyethylene and polypropylene. More specialized polyolefins include polyisobutylene and polymethylpentene. They are all colorless or white oils or solids. Many copolymers are known, such as polybutene, which derives from a mixture of different butene isomers. The name of each polyolefin indicates the olefin from which it is prepared; for example, polyethylene is derived from ethylene, and polymethylpentene is derived from 4-methyl-1-pentene. Polyolefins are not olefins themselves because the double bond of each olefin monomer is opened in order to form the polymer. Monomers having more than one double bond such as butadiene and isoprene yield polymers that contain double bonds (polybutadiene and polyisoprene) and are usually not considered polyolefins. Polyolefins are the foundations of many chemical industries.

Hot-melt adhesive (HMA), also known as hot glue, is a form of thermoplastic adhesive that is commonly sold as solid cylindrical sticks of various diameters designed to be applied using a hot glue gun. The gun uses a continuous-duty heating element to melt the plastic glue, which the user pushes through the gun either with a mechanical trigger mechanism on the gun, or with direct finger pressure. The glue squeezed out of the heated nozzle is initially hot enough to burn and even blister skin. The glue is sticky when hot, and solidifies in a few seconds to one minute. Hot-melt adhesives can also be applied by dipping or spraying, and are popular with hobbyists and crafters both for affixing and as an inexpensive alternative to resin casting.

Fluorinated ethylene propylene (FEP) is a copolymer of hexafluoropropylene and tetrafluoroethylene. It differs from the polytetrafluoroethylene (PTFE) resins in that it is melt-processable using conventional injection molding and screw extrusion techniques. Fluorinated ethylene propylene was invented by DuPont and is sold under the brandname Teflon FEP. Other brandnames are Neoflon FEP from Daikin or Dyneon FEP from Dyneon/3M.

A blister pack is any of several types of pre-formed plastic packaging used for small consumer goods, foods, and for pharmaceuticals.
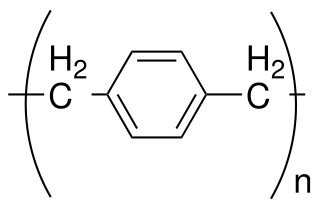
Parylene is the common name of a polymer whose backbone consists of para-benzenediyl rings −C
6H
4− connected by 1,2-ethanediyl bridges −CH
2−CH
2−. It can be obtained by polymerization of para-xylyleneH
2C=C
6H
4=CH
2.

Polymethylpentene (PMP), also known as poly(4-methyl-1-pentene), is a thermoplastic polyolefin. It is used for gas-permeable packaging, autoclavable medical and laboratory equipment, microwave components, and cookware. It is commonly called TPX, which is a trademark of Mitsui Chemicals.

A plastic bottle is a bottle constructed from high-density or low density plastic. Plastic bottles are typically used to store liquids such as water, soft drinks, motor oil, cooking oil, medicine, shampoo or milk. They range in sizes, from very small bottles to large carboys. Consumer blow molded containers often have integral handles or are shaped to facilitate grasping.

Ethylene vinyl alcohol (EVOH) is a formal copolymer of ethylene and vinyl alcohol. Because the latter monomer mainly exists as its tautomer acetaldehyde, the copolymer is prepared by polymerization of ethylene and vinyl acetate to give the ethylene vinyl acetate (EVA) copolymer followed by hydrolysis. EVOH copolymer is defined by the mole % ethylene content: lower ethylene content grades have higher barrier properties; higher ethylene content grades have lower temperatures for extrusion.

Cyclic olefin polymer (COP) is a type of amorphous polymer used in a wide variety of applications including pharmaceutical packaging and medical devices, optics and displays, and electronics.

Twin-wall plastic, specifically twin-wall polycarbonate, is an extruded multi-wall polymer product created for applications where its strength, thermally insulative properties, and moderate cost are ideal. Polycarbonate, which is most commonly formed through the reaction of Bisphenol A and Carbonyl Chloride, is an extremely versatile material. It is significantly lighter than glass, while managing to be stronger, more flexible, and more impact resistant. Twin-wall polycarbonate is used most commonly for green houses, where it can support itself in a structurally sound configuration, limit the amount of UV light due to its nominal translucence, and can withstand the rigors of daily abuse in an outdoor environment. The stagnant air in the cellular space between sheets provides insulation, and additional cell layers can be extruded to enhance insulative properties at the cost of light transmission.

Cyclohexanedimethanol (CHDM) is a mixture of isomeric organic compounds with formula C6H10(CH2OH)2. It is a colorless low-melting solid used in the production of polyester resins. Commercial samples consist of a mixture of cis and trans isomers. It is a di-substituted derivative of cyclohexane and is classified as a diol, meaning that it has two OH functional groups. Commercial CHDM typically has a cis/trans ratio of 30:70.

In polymer chemistry, graft polymers are segmented copolymers with a linear backbone of one composite and randomly distributed branches of another composite. The picture labeled "graft polymer" shows how grafted chains of species B are covalently bonded to polymer species A. Although the side chains are structurally distinct from the main chain, the individual grafted chains may be homopolymers or copolymers. Graft polymers have been synthesized for many decades and are especially used as impact resistant materials, thermoplastic elastomers, compatibilizers, or emulsifiers for the preparation of stable blends or alloys. One of the better-known examples of a graft polymer is a component used in high impact polystyrene, consisting of a polystyrene backbone with polybutadiene grafted chains.



















